LOOK A-LIKE SOUND A-LIKE (LASA) DRUGS: A MULTIFACETED CHALLENGE
"Look a-like, sound a-like" (LASA) refers to a significant issue within the pharmaceutical industry where medications bear similar names or appearances but differ in their active ingredients, dosage forms, strengths, or indications. Look-alike medicines appear visually the same with respect to packaging, shape, colour and/ or size, while sound-alike medicines are similar in the phonetics of their names, doses and/or strengths. These similarities can lead to confusion among healthcare providers, pharmacists, and patients, potentially resulting in medication errors that compromise patient's health.
LASA incidents highlight the critical importance of clear labelling, distinctive packaging, and effective communication strategies to prevent misunderstandings and ensure the correct administration of medications. LASA errors can occur at any stage of medication use: prescribing, transcribing or documenting, dispensing, administering and monitoring.
LASA occurrences are a global concern, prompting regulatory agencies to implement stringent guidelines to mitigate risks. These measures include requirements for unique drug names, standardized labelling formats, and rigorous pharmacovigilance practices to monitor and address potential issues promptly. Healthcare professionals play a crucial role in identifying and reporting LASA incidents to enhance patient safety and improve medication management practices.
This newsletter delves into the multifaceted issue of "Look-alike, Sound-alike" (LASA) drugs, exploring how similarities in drug names and appearances can lead to medication errors. We examine the impact of LASA drugs on patient safety, regulatory challenges, and the role of intellectual property in the pharmaceutical industry. The article provides an overview of recent regulatory efforts in India, judicial decisions to tackle the issues with LASA drugs, and the need for comprehensive solutions, including technological interventions and enhanced pharmacovigilance. Through this analysis, we aim to highlight ongoing challenges and propose actionable strategies to mitigate the risks associated with LASA drugs.
LASA Drug Issues and Trademark Law
In the pharmaceutical sector, Intellectual Property (IP) rights play a crucial role in incentivizing innovation and protecting investments in research and development. IP encompasses patents, trademarks, copyrights, and trade secrets that enable pharmaceutical companies to safeguard their innovations, brand identities, and market positions.
LASA issues in the pharmaceutical industry intersect with trademark laws that ensure that brand names of medicines are distinguishable. To avoid confusion and potential medication errors, pharmaceutical companies must ensure that their trademarks, product names, and packaging are distinctly different from those of competitors. This requires not only registering and defending trademarks but also carefully designing and reviewing brand elements. Effective management of these IP aspects helps prevent legal disputes, maintains clear market presence, and ensures that patients can easily identify and trust the medications they use.
LASA ISSUES IN INDIA'S PHARMACEUTICAL LANDSCAPE
The issue of "Look alike, sound alike" (LASA) is particularly significant in India's pharmaceutical landscape due to several reasons:
- Diverse Market Dynamics:India has a vast pharmaceutical market with a wide range of medications available from numerous manufacturers. This diversity increases the likelihood of similar-looking or sounding drug names, heightening the risk of medication errors.
For example, GNAP-10 (an anti-hypertensive medication), generic Telmisartan (an anti-hypertensive medication), Glimeimore (an oral hypoglycemic agent), and generic Metoprolol tartrate (a beta-blocker) are manufactured as round white tablets and have almost the same size, which poses the risk of confusion among consumers and adverse health consequences.
- Patient Safety:LASA errors can have severe consequences for patient safety. In a country where healthcare access and literacy vary widely, confusion over medication names or appearances can lead to incorrect dosages or treatments, impacting patient health outcomes.
For example, the brand, IMOX for amoxicillin tablets for humans and INIMOX for a combination of amoxicillin and cloxacillin10 as an injection for veterinary use. Since there are many brands of amoxicillin + cloxacillin combination in tablet form and for human use, it is possible that a pharmacist will not just dispense but also administer this injection when a doctor prescribes either amoxicillin or the amoxicillin + cloxacillin combination tablets.
- Regulatory Challenges: Ensuring stringent regulatory oversight to prevent LASA errors in India is particularly challenging due to the market's complexity. The Indian pharmaceutical sector is vast and rapidly evolving, with a high volume of drug names and varied regional practices.
This complexity complicates the enforcement of consistent standards for drug naming, packaging, and labeling. Effective oversight requires robust collaboration between regulatory bodies and pharmaceutical companies to develop and implement clear, standardized guidelines.
The dynamic nature of the market, along with frequent changes and the introduction of numerous new products, demands continuous vigilant monitoring to mitigate LASA risks and ensure patient safety.
- Impact on Healthcare Professionals:LASA errors not only affect patients but also place a burden on healthcare professionals who must navigate potentially confusing drug names and packaging. Clear differentiation in product presentation is essential to support accurate prescription and administration practices.
- Intellectual Property Considerations:LASA issues also intersect with intellectual property rights, as pharmaceutical companies strive to protect their brand identities and prevent infringement. Clear differentiation in trademarks and brand strategies is essential to mitigate legal disputes and maintain market integrity.
OVERVIEW OF RECENT REGULATORY EFFORTS
Recent developments in India's pharmaceutical regulatory landscape highlight significant efforts to improve patient safety and address the longstanding issue of look-alike and sound a-like (LASA) drugs. This initiative follows a series of recommendations and actions taken by the Drug Technical Advisory Board (DTAB) and the Director General of Health Services (DGHS).
In January 2024, the Drug Technical Advisory Board (DTAB) recommended a prohibition on the marketing and the manufacturing of different drugs under the same brand name.1 This decision stemmed from the ongoing concerns regarding the potential health risks associated with drug name confusion, which can lead to medication errors and adverse drug reactions. Following these recommendations, the Director General of health Services (DGHS) took a proactive approach and wrote a letter to the Controller General of Patents Designs and Trademarks (CGPSD). In this letter he recommended to enhance the oversight and implementation of stricter trademark regulations for pharmaceutical products. This initiative was prompted by ongoing concerns regarding the serious health risks associated with confusion arising from similar drug names.2
Despite these proactive measures, several challenges still remain. Ensuring compliance among pharmaceutical companies regarding the prohibition of identical brand names will require rigorous monitoring and enforcement mechanisms. The effectiveness of these regulations will depend on the capacity of regulatory bodies to oversee and address violations. Furthermore, there is a pressing need for increased public awareness about the risks associated with LASA drugs, as patients, especially those within limited literacy, often rely on visual cues for medication identification.
Without proper education and resources, several issues arise regarding drug safety and efficacy:
- Poor enforcement and oversight – There is a heightened risk of inadequate enforcement and oversight in the pharmaceutical sector, leading to gaps in the regulatory process. This lack of oversight can result in non-compliance with established safety standards and regulations, putting public health at risk.
- Inadequately trained Pharmacists – Many pharmacists are poorly trained which impacts their ability to safely dispense medications and provide proper guidance to the patients. Inadequate training can lead to errors in medication administration, miscommunication with healthcare providers, and a general decrease in the quality of care.
- Lack of robust Frameworks – The absence of strong frameworks further complicates the management of drug safety and efficacy. Without clear guidelines and procedures, it becomes challenging to monitor and ensure that drugs meet necessary safety standards throughout their life cycle.
It is pertinent to note that, even though the recent actions taken by the DTAB and DGHS represent a significant step towards improving drug safety in India, the success of these initiatives will heavily depend on effective implementation, public education, and a robust regulatory framework to address the ongoing challenges in the pharmaceutical sector.
The need for a centralized drug name database
A critical yet often overlooked aspect of addressing the LASA drug issue is the establishment of a centralised database of drug names. The National Health Authority of India's consultation paper on Drug Registry suggests that such a database is a solution.3 This resource would enable pharmacists, healthcare providers and regulators to cross-reference drug names efficiently and accurately, significantly reducing the risk of confusion and medication errors.
Currently the absence of such a database hampers effective regulations and places undue burden on healthcare professionals who are on the front lines of dispensing medications. Without a comprehensive resource to identify potential conflicts in drug naming, the risk of confusion remains high, posing serious risks to patient safety.
Judicial Decisions tackling issues related to LASA drugs
A trademark serves as a distinctive sign that identifies and distinguishes the source of goods or services, thereby ensuring that consumers can make informed choices about the medications they use. In the context of pharmaceuticals, trademarks are particularly significant because they prevent confusion among healthcare professionals and patients, especially for those who may not be able to read or understand drug labels. For such patients, the visual characteristics of a drug—its shape, color, and packaging—become critical identifiers. Therefore, similar-looking drugs with misleading names can lead to dangerous medication errors.
It is a general observation that most of the medicine names are based on their International NonProprietary Names. The incorporation of International Nonproprietary Names (INNs) into pharmaceutical trademarks raises complex legal and ethical issues. Section 13 of The Trade Marks Act, 1999 (India), prohibits the registration of trademarks that consist of or resemble INNs, emphasizing that these names should remain public property and not be appropriated by pharmaceutical companies.
CADILA HEALTHCARE LIMITED V. CADILA PHARMACEUTICALS LTD - FALCIGO AND FALCITAB
Judicial interpretations of trademark law have become increasingly important in addressing challenges with LASA drugs. Courts are tasked with balancing the protection of trademark rights with the need to uphold public health standards. In the landmark case of Cadila Healthcare Limited v. Cadila Pharmaceutical Ltd, (2001) 5 SCC 73, the Supreme Court of India has held that strict measures are required to be taken to prevent any confusion arising from similarity of trademarks in pharmaceutical products. The Apex Court has acknowledged that factors that the drug is sold under prescription or only to physicians are not sufficient to prevent confusion amongst deceptively similar trademarks, which is otherwise likely to occur. Even though physicians and pharmacists are trained people, a possibility of mistake by them may prove to be fatal. This case set an important precedent advocating for a stricter regime that meticulously evaluates drug names to avoid confusion, highlighting the crucial role of enforcing trademark law in pharmaceutical branding.
SUN PHARMACEUTICAL LABORATORIES LTD. V. HETERO HEALTHCARE LTD. LETROZ AND LETERO
However, recent court decisions regarding trademarks, such as in the case of Sun Pharmaceutical Laboratories Ltd. V. Hetero Healthcare Ltd and Anr, 2022 SCC OnLine Del 939, disregards the similarity of the concerned trademarks 'Letroz' and 'Letero' on the assumption that healthcare professionals' expertise and prescription mechanisms are sufficient to mitigate confusion. This assumption is problematic as it underestimates the potential for human error and overlooks the Supreme Court's cautions against such reliance. In practice, minor oversights can result in significant health risks, emphasizing the need for robust regulatory mechanisms.
The Indian Courts have been delivering inconsistent and conflicting decisions when it comes to deciding where the LASA drugs are infringing trademark laws. Such decisions leave it upon pharmaceutical companies to fight their own trademark infringement battles to tackle the issue of deceptively similar brand names. Thus, it is important for the drug regulatory body to grant marketing authorisation for a drug only after reviewing the trademark search report so that the issue of misleading brand names does not arise.
The role of visual elements in drug identification
Visual elements like packaging, colour and design plays a significant role in drug identification, a factor often overlooked in pharmaceutical regulations. In other industries, trademarks and distinctive packaging are vital for brand identification. However, in pharmaceuticals, these elements have a far reaching implications. The strong recall value associated with specific colours, shapes, and packaging designs can significantly impact the patient's health.
This issue was highlighted in the case of Glaxo Wellcome v. Sandoz & Others (2019) EWHC 2845 (Ch). In October 2019, Lord Justice Arnold dismissed GlaxoSmithKline's (GSK) High Court claim against Sandoz, concerning Sandoz's AirFluSal Forspiro inhaler, regarding passing off by mimicking the color and appearance of GSK's Seretide Accuhaler. After the expiration of GSK's patent for Seretide's active ingredients, Sandoz launched the AirFluSal Forspiro as a competitor. GSK argued that the similarities in color and appearance falsely implied that AirfluSal Forspiro was equivalent to the Seretide Accuhaler. The Court however, found no evidence that the purple color used by Seretide had become distinctive to GSK or Seretide, and no proof of patient confusion regarding the origin or equivalence of the inhalers.
Given below is the comparison between both the products –
GLAXO WELLCOME V. SANDOZ & OTHERS - GSK AND SANDOZ
The decision underscores the need for a balanced approach that considers both trademark rights and public health needs. In pharmaceuticals, the implications of visual similarities are far more consequential, potentially affecting patient safety and treatment efficacy.
COMPREHENSIVE SOLUTIONS
To mitigate the risks associated with look-alike and sound-alike (LASA) drugs, a technical and multifaceted approach is essential. This approach encompasses leveraging technology, enhancing regulatory frameworks and improving educational outreach.
- Data Collection and Analysis : One of the critical issues in India is the lack of data on prescription errors, leading the Ministry of Health to mistakenly assume that no data means no problem. Recognizing this issue is essential for initiating reforms similar to those in the United States and Europe [1], where dedicated divisions within drug regulatory bodies assess drug names on various factors to prevent confusion and reduce prescription errors.
- Drug level Policy : In order to minimize confusion and ensure patient safety, it is essential to permit unique brand names for innovator drugs, as is currently practiced in the USA. Alongside this, the compulsory use of International Nonproprietary Names (INN) should be emphasized prominently on packaging, along with the specific indication for which the drug is prescribed. Drugs with misleading names should be withdrawn immediately to prevent errors. Additionally, uniformity in the shapes, colors, and designs of specific types of drugs is crucial, particularly where misuse could lead to serious adverse effects. There should also be stringent enforcement of pharmacovigilance obligations, including tracking prescription and dispensing errors. To further support this effort, regular updates and advisories should be provided on prescription and dispensing errors, as well as a list of drugs with misleading names.
- Technological Interventions : Incorporating phonetic and orthographic analysis tools during the approval process can significantly reduce the risk of approving LASA (Look-Alike, Sound-Alike) drugs. The Food and Drug Administration (FDA) of the USA [2] has successfully used its Phonetic and Orthographic Computer Analysis (POCA) tool to evaluate potential drug name confusions before they enter the market, providing a model for India to consider.
- Regulatory Enhancements : Strengthening drug labelling and packaging regulations is essential. Clear and distinctive labelling can help differentiate drugs, with regulations requiring manufacturers to use unique colour schemes, font sizes, and packaging shapes to minimize LASA (Look-Alike, Sound-Alike) errors. Implementing uniform colour schemes for drug identification can also help reduce medication errors. By standardizing color-coding across the industry for drugs that treat similar conditions or contain the same active ingredients, healthcare providers and patients can easily identify the correct medication, thereby improving adherence to prescribed treatments.
One commonly used method for distinguishing LASA medication pairs is 'Tall – Man Letters' (TML). Following are a few examples of established drug names recommended to use tall man lettering :
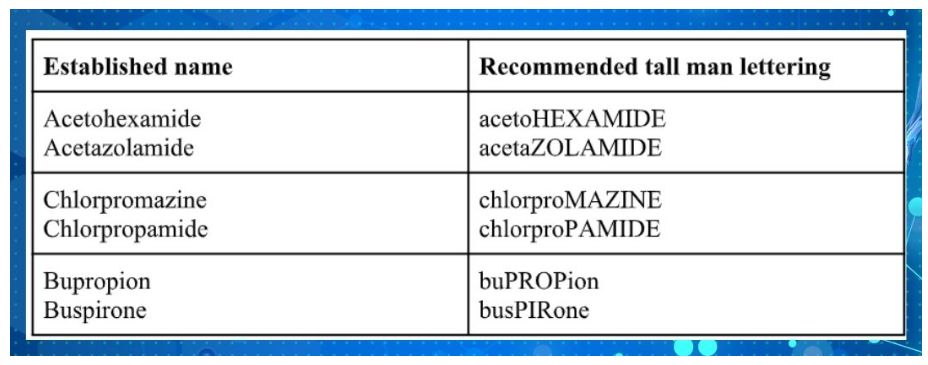
TML helps differentiate LASA medication names by using uppercase letters for certain parts of the name. This technique is often applied to syllables or groups of letters within a medication name to emphasize the differences between confusing names.
- Consistent Judicial Approach and Trademark Search before giving approval of the medicine name: It is important for the drug regulatory body to grant marketing authorisation for a drug only after reviewing the trademark search report so that the issue of misleading brand names does not arise. It is also important for the Indian Courts to prevent the confusing brand names by giving consistent and uniform decisions. It is also important for pharmaceutical companies to keep a watch on the Trademark journal and the indian market for taking swift action against LASA drugs for safeguarding their credibility as well as the patient's health.
- Pharmacovigilance Strengthening : Another crucial recommendation is to enhance pharmacovigilance systems to monitor and address LASA-related issues after marketing. Robust systems should be capable of tracking adverse drug reactions and other safety concerns associated with LASA drugs, enabling swift regulatory action and efficient information dissemination to healthcare providers.
- Education and Training : A comprehensive regulatory reform is essential, along with training for healthcare professionals and pharmacists on Look-Alike Sound-Alike (LASA) medications. More stringent controls and regular audits should be instituted to ensure adherence to drug naming and labelling standards. Continuous professional development focusing on the latest regulatory updates and safety protocols is critical for minimizing dispensing errors. By implementing these recommendations, India can make substantial progress in tackling LASA drug issues, ultimately improving patient safety and healthcare outcomes.
- Short-term and immediate solution : The only short term and immediate solution to the issue of LASA (Look Alike and Sound Alike) drugs is for doctors to adhere to guidelines requiring them to write the International Nonproprietary Names (INN) of drugs in capital/bold letter in the prescriptions. As per the observation of the Orissa High Court in the case of Rasa @ Rasananda Bhoi vs State of Odisha & Ors., W.P.(C) No. 38461 of 2023, the Odisha government emphasized the National Medical Commission (NMC) guidelines and instructed all doctors in both government and private institutions to write legible prescriptions. However, while legible handwriting is a part of the solution, it only partially addresses the problem. Emphasizing the writing of INNs in prescriptions is crucial, as it helps pharmacists confirm and dispense the correct medicines. Though this measure may not completely eliminate prescription and dispensing errors, it is an important step in the right direction .
CONCLUSION
Collaboration between regulatory agencies, medical experts, pharmaceutical companies, and the community is essential as India moves forward in tackling LASA medication issues. To safeguard patient safety and enhance the quality of healthcare, these reforms must be not only put into place but also strictly enforced and updated on a regular basis.
The recent initiatives implemented by the Director General of Health Services (DGHS) and ongoing discussions by the Drugs Consultative Committee (DCC) are encouraging moves toward changing drug nomenclature and prescribing procedures.
To ensure effectiveness, these programs must be part of a larger, long-term effort that involves rigorous monitoring and evaluation.
Furthermore, integrating technology, such as digital platforms and mobile apps, can improve patient engagement while also providing healthcare providers with tools for effectively identifying and managing LASA medicines. The construction of a centralized database of medicine names, as well as the use of phonetic and orthographic analytic techniques, can help to greatly reduce medication errors.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


