In the world of patent law, clarity is key. Building on my takeaways from the In re Cellect decision last year, a recent Federal Circuit case, Allergan USA, Inc. v. MSN Laboratories Private Ltd., No. 2024-1061 (Fed. Cir. August 13, 2024), provides essential insights into patent law, offering valuable lessons for any patent attorney. Here's an overview of the case, key insights, and its implications for patent law.
Executive Summary
Key Clarification: This case clarifies when a patent claim can act as an obviousness-type double patenting (ODP) reference, pivotal for patent lawyers.
Decision Impact: The ruling asserts that a later-filed, later-issued, earlier-expiring patent ('011 Patent) cannot invalidate an earlier-filed, earlier-issued, later-expiring patent ('356 Patent) sharing the same priority. This decision holds significant implications for any patent attorney managing patent portfolios.
Open Question for Patent Attorneys
Unresolved Issue: What if the parent case ('356 patent) issued after the child ('011 patent)?The Court's interpretation of the "first patent" is crucial, yet seemingly sidestepped in the decision. Determining the "first patent" by filing or issuance date influences the potential implications significantly. If the '356 patent qualifies as the first based on its filing date, the current holding should remain unchanged. Conversely, if the '011 patent is identified as the first due to its issuance date, the '011 patent could establish the maximum allowable time for the invention – a critical consideration for any patent attorney.
Comprehensive Overview
In Allergan USA, Inc. v. MSN Laboratories Private Ltd., No. 2024-1061 (Fed. Cir. August 13, 2024), the Federal Circuit held that a first-filed, first-issued, later-expiring claim cannot be invalidated by a later-filed, later issued, earlier-expiring reference claim having a common priority date. That is, the first filed, first-issued patent in its family sets the maximum period of exclusivity for the claimed subject matter and any patentably indistinct variants.
Facts
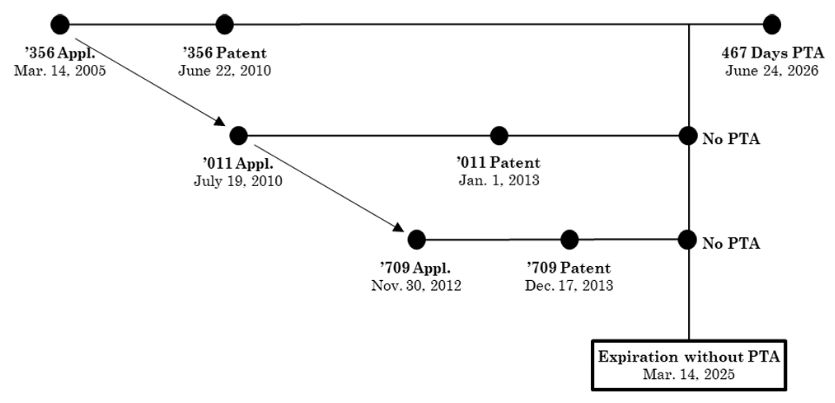
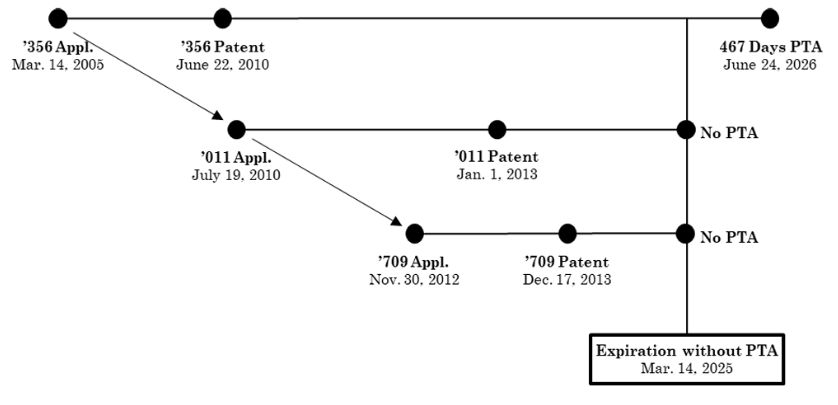
In 2015, the U.S. Food and Drug Administration (FDA) approved the New Drug Application (NDA) for eluxadoline tablets, marketed and sold by Allergan under the brand name Viberzi®. Viberzi is protected by several issued patents. The first patent application for this product was filed on March 14, 2005, leading to the issuance of the '356 patent on June 22, 2010.
Due to prosecution delays, the '356 patent received a patent term adjustment (PTA), extending its expiration to June 24, 2026, beyond the standard 20-year term from its priority date. Several continuing applications claiming priority from the March 14, 2005, filing date of the '356 patent, were also filed. Notably, U.S. Patents 8,344,011 (the '011 patent) and 8,609,709 (the '709 patent) are relevant here. The chart below details their respective filing, issuance, and expiration dates. Since there was no prosecution delay, the '011 and '709 patents did not receive any PTA and will expire on March 14, 2025, twenty years from its earliest priority date.
In July 2019, Sun submitted an Abbreviated New Drug Application (ANDA) seeking FDA approval to market and sell a generic version of Viberzi. Allergan sued Sun, claiming that Sun's ANDA filing directly infringed claim 40 of the '356 patent.
District Court's Decision
Before the trial, both parties stipulated that Sun would infringe all asserted claims if those claims were valid. The key issue in the three-day bench trial was whether claim 40 of the '356 patent was invalid due to ODP. Sun argued that claim 40 was invalid for ODP over claim 33 of the '011 patent and claim 5 of the '709 patent, as the claims were not patentably distinct and claim 40, with 467 days of PTA, expired after the reference claims of the '011 and '709 patents.
Allergan countered that since the '356 patent was the first to claim eluxadoline tablets and the first to issue, claim 40 should not be subject to ODP over the later-filed, later-issued reference patents. The district court sided with Sun, dismissing Allergan's "first-filed, first-issued" argument as "immaterial." The court ruled that ODP analysis focuses on patent expiration dates, not filing or issuance dates, citing Gilead Scis., Inc. v. Natco Pharma Ltd., 753 F.3d 1208, 1215–17 (Fed. Cir. 2014), and In re Cellect, LLC, 81 F.4th 1216, 1228–29 (Fed. Cir. 2023). Allergan appealed.
Federal Circuit's Holding
The appeal questioned whether a first-filed, first-issued, later-expiring claim can be invalidated by a later-filed, later-issued, earlier-expiring reference claim with a common priority date. The Federal Circuit ruled that it cannot.
The Court distinguished this case from In re Cellect. In that case, the Court held that in ODP analysis the relevant expiration date includes the PTA, not the original expiration date. Apply that decision to the facts of this case results in the '356 patent's expiration date being June 24, 2026, including PTA. However, this doesn't mean that the '356 patent is invalid over the '011 and '709 patents for expiring later. The Court noted In re Cellect doesn't address when a claim can serve as an ODP reference.
The Court concluded that the '011 and '709 patents can't invalidate claim 40 of the '356 patent. This aligns with the ODP doctrine's purpose. The doctrine prevents extending a patent's life via a second patent on a similar invention. The '356 patent is the first to cover eluxadoline tablets, regardless of filing or issuance date. The '011 and '709 patents claim priority from it. The '356 patent's claims don't extend the monopoly on eluxadoline tablets. They remain within the legal period and aren't subject to ODP over the '011 and '709 patents.
The fact that the '356 patent expires later is irrelevant. It isn't a second, later-expiring patent for the same invention. The Court held that, as the first-filed and first-issued patent, it sets maximum exclusivity. This applies to the claimed subject matter and any patentably indistinct variants. Therefore, a first-filed, first-issued, later-expiring claim can't be invalidated this way. A later-filed, later-issued, earlier-expiring reference claim with a common priority date doesn't apply.
Conclusion
This case underscores the intricacies of patent filing and expiration dynamics, offering much-needed clarity on ODP references. For any patent attorney, this case reinforces the importance of understanding patent timelines and their implications on exclusivity periods. As we continue to navigate these complex legal landscapes, staying informed is essential for effective patent strategy and protection.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


