On Aug. 7, 2024, the Centers for Medicare and Medicaid Services (CMS) finalized a new rule to expedite Medicare coverage of certain FDA-designated breakthrough devices known as the Transitional Coverage for Emerging Technologies (TCET) Pathway. In a fact sheet, CMS stated that through the TCET, it aims to improve the care and quality of life for people with Medicare, while enhancing and encouraging innovation.
CMS limits the TCET Pathway to five breakthrough devices per year and excludes digital therapeutics (as it requires technologies to come under an existing Medicare benefits category). Further, for technologies accepted into, and continuing in, the TCET Pathway, the agency's goal is to finalize a national coverage determination (NCD) within six months after FDA market authorization, which would last for five years.
The TCET replaces a prior final rule released under the Trump administration in January 2021 known as the Medical Coverage of Innovative Technology (MCIT) pathway, in which CMS would provide immediate Medicare coverage for FDA-designated breakthrough devices lasting for a four-year transitional period from the date of FDA market authorization (or a manufacturer chosen date within two years thereafter). However, soon after President Biden took office, his administration repealed the MCIT Pathway due to alleged safety and clinical benefit concerns.
TCET Pathway
CMS has released the following TCET pathway diagram for guidance:
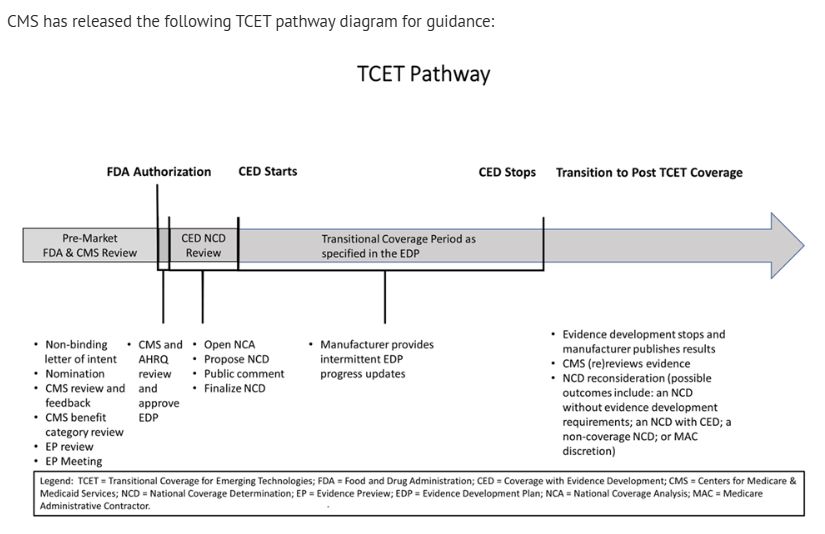
The TCET pathway intends to balance multiple considerations when making Medicare coverage determinations: (1) facilitating early, predictable, and safer access for people with Medicare to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes.
CMS states that the TCET pathway would apply to certain eligible FDA-designated breakthrough devices, as it has determined this is the area with the most immediate need for a pathway like TCET. Manufacturers of such devices may self-nominate to participate in the TCET pathway, ideally approximately 12 months before the manufacturer anticipates an FDA decision on a submission.
Bipartisan Legislation
In June 2024, the House Ways and Means Committee advanced bipartisan legislation, Ensuring Patient Access to Critical Breakthrough Products Act of 2023, which mirrors the MCIT Pathway in allowing FDA-designated breakthrough devices to be immediately covered under Medicare during a four-year transitional period while CMS makes a NCD. The program coverage would take effect 18 months after enactment. During this period, CMS would need to assign payment codes for such devices within three months of FDA approval and establish a process to allow for continued coverage after the transitional period has expired. The bill also includes additional safety standards for FDA-designated breakthrough devices, such as mandating that the devices have clinical trial information on the Medicare population. GT will continue tracking these movements.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



