- within Insolvency/Bankruptcy/Re-Structuring topic(s)
- with readers working within the Insurance industries
As many have now heard, on Thursday, August 15, 2024, the Centers for Medicare & Medicaid Services (CMS) released the prices for the first 10 prescription drugs selected for negotiation with manufacturers (Selected Drugs) under the Inflation Reduction Act of 2022 (IRA) Medicare Drug Price Negotiation Program (Price Negotiation Program). As explained in a previous alert, the Price Negotiation Program allows CMS to directly negotiate drug prices for certain high expenditure, single source Medicare Part B and Part D drugs on an escalating scale (additional details regarding the Price Negotiation Program can be found here). CMS announced the ten Part D Selected Drugs for the initial price applicability year 2026 in August 2023.
In January 2024, CMS and the manufacturers of the 10 Part D Selected Drugs began the Price Negotiation Program process, with negotiations concluding earlier this month. CMS took into consideration information provided by manufacturers when determining the new discounted prices, which included research and development costs, prior federal financial support, unit costs of production and distribution, and market/revenue/sales data.
As a result, CMS arrived at the following prices for the 10 Part D Selected Drugs, effective January 1, 2026:
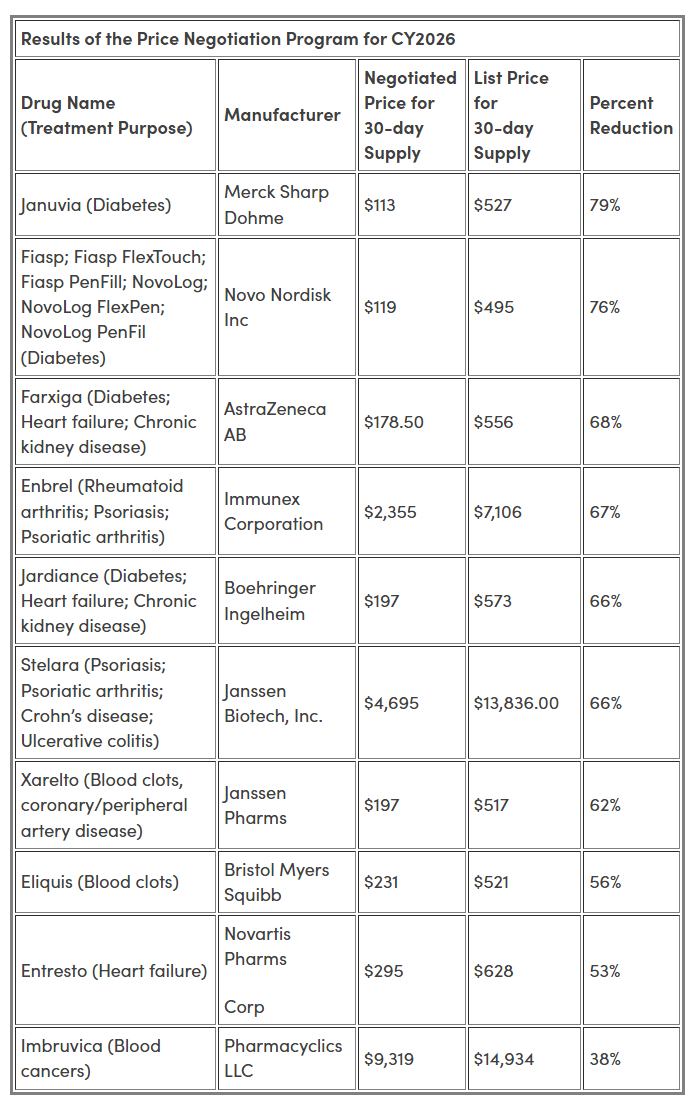
The negotiated prices range from 38% to 79% discounts off the 30-day supply list prices. In a press release, CMS commented that if the new prices had been in effect last year, Medicare would have saved an estimated $6 billion, or approximately 22%, across the 10 Selected Drugs.
What happens next?
- CMS has until March 2025 to publish an explanation for the negotiated prices for each Selected Drug that go into effect on January 1, 2026.
- By February 2025, CMS will select up to 15 more drugs covered under Part D for negotiations for 2027. Drug manufacturers will have until the end of that month to decide whether to participate in the Price Negotiation Program.
- Following the second round of price negotiations, CMS may negotiate prices for another 15 drugs that will go into effect in 2028, and then another 20 drugs that will go into effect in 2029.
What actions should you take now?
As a result of the Price Negotiation Program and the drug pricing changes to be expected over the next few years, health plans are encouraged to review closely the financial terms of their pharmacy benefit management (PBM) agreements to consider whether the new negotiated 2025 drug prices trigger certain rights or actions under the agreements (e.g., reservation of rights, exception events, equitable adjustment, changes to law, etc.) that permit the PBM or health plan to adjust the drug pricing or financial guarantees beginning in 2025.
As CMS continues to negotiate new drug prices under the Price Negotiation Program, health plans should anticipate the impact of these price fluctuations during the term of a current (or future) agreement and determine the methodology and process to be used for accessing the impact of these changes.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



