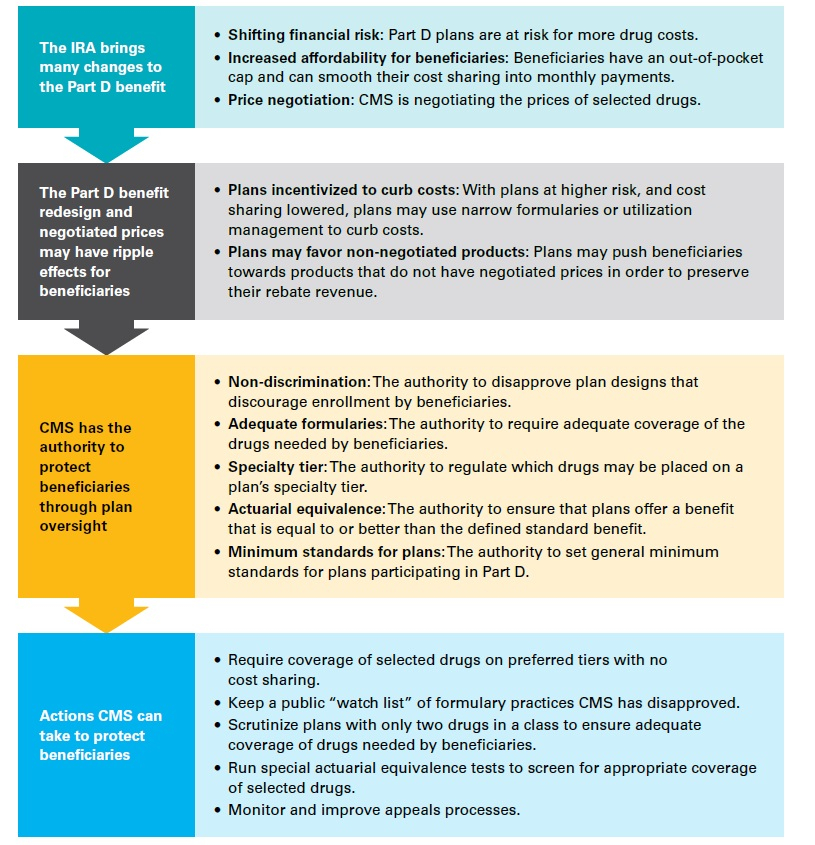
As part of IRA implementation and in advance of calendar year 2025, which is when the implementation of Part D redesign changes accelerate, CMS could take steps to protect beneficiaries by preserving access and increasing transparency. CMS has already warned PDP sponsors not to place drugs selected for negotiation on non-preferred formulary tiers without clinical justification. But it can do more under its current legal authority.
This paper discusses in detail the coming changes to Part D, some of the risks faced by beneficiaries as a result of plan sponsors' changing incentives based on the IRA, the initial measures taken by CMS to date to protect beneficiaries, and steps CMS could take under its current legal authority to better protect timely beneficiary access to therapy.
Download Insight to read the full report.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


