A recent report from the European Patent Office (EPO), here, revealed that patents for mRNA technologies, which played a pivotal role in mitigating the impact of COVID-19, have increased at an accelerated rate in recent years. The report further highlights that the "above average" number of international applications made through the Patent Cooperation Treaty reflects "multinational commercialisation strategies" and thus serves as a proxy for the "high economic expectation" for these life-saving technologies.
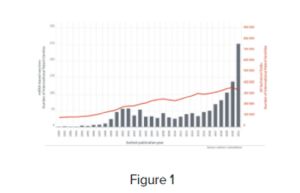
The report identified 2,300 inventions (defined by the number of unique patent families) related to mRNA vaccines, pointing out the rate of increase in recent decades. Although mRNA vaccines were globally approved for medical use only in recent years, mRNA-based drug development has been studied since the 1980's. Since then, there has been a steady growth in the number of inventions; in 1990, approximately 25 patents were filed compared to around 350 in 2021. This trend is reflected in Figure 1 of the report, which shows that the patent filings for mRNA technologies has outstripped the baseline rate across all technological fields. The insight report observes that pressure to "innovate at speed" during the COVID-19 pandemic alongside decades of research which gradually improved the design and delivery of mRNA vaccines, facilitated the dramatic increase in inventions and patents in recent years.
Moreover, the report revealed that WIPO received the highest proportion of patent applications under the Patent Cooperation Treaty, out of all multi-jurisdictional patent filings. This indicates a desire for widespread geographical reach and the EPO suggests that inventions related to mRNA vaccines are perceived as holding a high economic potential. The increasing use of international patent authorities when seeking to file mRNA technologies patents demonstrates that "multinational commercialisation strategies" are being adopted by companies hoping to exploit patents globally.
The report also outlines the key jurisdictions and companies from where patent applications were originating, highlighting "the key role of applicants from the United States, Europe and China". Their analysis of international databases revealed that the top 20 patentees were based in one of these jurisdictions, with 13 coming from the United States alone. It also highlighted that Moderna topped the list for most patent filings, filling 96 applications in 11 years, with CureVac coming in a close second at 95 applications. These findings are compatible with what is reflected in the patent litigation landscape, with both patentees recently engaging in global litigation to assert their various patents.
Indeed, the EPO report provides a valuable lens into the 'patent wars' which have emerged around mRNA vaccines in the last years. As indicated in the report, mRNA-related technology holds "high economic expectation" and therefore has become the subject of various intellectual property disputes. Moderna's infringement proceedings against Pfizer and BioNTech was one of the first to catch the headlines, with CureVac's claims against the same following soon after. The increasing value being attributed to mRNA vaccine inventions and its future potential in medical therapy provides a clear insight into why it is becoming such a contentious field of intellectual property. As demonstrated by the EPO report, the "field of mRNA-based vaccines is very dynamic and the momentum in this field is clearly above average". Patent exploitation will be key to marking out the various players' claim to the mRNA market.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


