Introduction
Antimicrobial resistance (AMR) is one of the biggest global threats and tackling it requires coordinated international action across the healthcare, agriculture and environmental sectors.
As previously reported (here), it is almost 10 years since the publication of the O'Neill report on antimicrobial resistance, which presented AMR as a critical global health threat that, if unchecked, could lead to 10 million deaths per year by 2050, with a $100 trillion economic cost. As well as increasing drug-resistance and antibiotic misuse, the report pointed to the lack of new antimicrobials, including antibiotics. The O'Neill report therefore provided a call to develop new antimicrobials, and noted that new economic incentives were required for the commercialisation of antimicrobials, particularly due to high development costs and low financial returns for new antimicrobials. However, has the call to action been realised? It is certainly the case that there has been a significant uplift in public and scientific interest, including articles investigating AMR and how to tackle it. Nevertheless, has there been a notable increase in the identification and development of new antimicrobials?
It is also 10 years since our Life Sciences Report (here) included a section looking at antibiotic patent filing statistics between 2004 and 2015. At that time, it was telling that when looking at the world's top 10 pharmaceutical companies, they were filing significantly fewer antibiotic patents as compared, for example, to rare disease medicine patents. Our data also showed that the number of new patent applications relating to antimicrobials remained steady over the period studied. As suggested in the O'Neill report, this points to the difficulty of bringing new antibiotics to market and being able to recoup the costs associated with commercialisation. O'Neill suggested the need for incentivisation, in order to encourage new antibiotic development.
Our Life Sciences Report additionally found that research into known classes of antibiotics was much greater than in respect to new antibiotic classes, with most patent applications being filed in relation to penicillins, aminoglycosides and macrolides, rather than other classes of antibiotics.
As it has been about a decade since both the O'Neill report and our Life Sciences Report were published, we decided to consider the patent filing statistics in this intervening period, in order to see what, if any, differences have occurred. Significant events have happened in that time, including most significantly having to contend with a global pandemic, which has alerted the world to the issues of the importance of global healthcare, and the perils of not having readily available medical treatments. Of course, the rapid development and commercialisation of vaccines to combat the pandemic is laudable, and we were intrigued to see if this has filtered across to the adjacent field of antimicrobials and their development, given their global importance.
Findings
A targeted search strategy was developed to identify patents relating to antimicrobials. Background research involved reviewing academic papers and online articles to gain a clear understanding of the field and its key terminology. The most commonly recurring terms and concepts were then identified and used to curate a selection of relevant keywords. These keywords were then combined with appropriate IPC classifications and searched using the Derwent Innovation patent database. This approach was carried out to ensure a more focused and comprehensive set of results, to allow for a clearer insight into trends and developments in patents relating to antimicrobial innovation.
Our following analysis looks at patent filings (i.e. published patent families) since 2015. For the purpose of this analysis, we looked at the number of patent families, rather than individual patent applications. We consider this to be more representative of the general desire by an applicant to seek patent protection for a given invention.
Examination of the raw data revealed that there were around 12,500 patent families with a priority date of 2015 or later. It should be noted that this value is dependent upon the search strategy employed. However, it is reasonable that, given the strategy adopted for this analysis, the results can be considered as representative of the following filing trends: the applicant; year-on-year evolution; and territory.
A more detailed review of the data is provided below.
Fig. 1: Number of published patent family applications with a priority date of 2015 onwards* (01.01.2015–28.10.2024)

*Most applications are not published until around 18 months from their earliest priority date. Therefore, the decrease from 2023 to 2025 most likely only reflects the normal publication delay.
The above caveat aside, the data suggests a steady increase in the number of global patent applications filed in the field of antimicrobials since 2015. A slight decrease can be observed in 2022, which may be due to a reduction in antimicrobial research immediately following the Covid-19 pandemic and a greater focus on vaccine development. The increase in antimicrobial patent filings is encouraging, as one might expect that with more filings, the greater the possibility of new antimicrobials coming to market. However, when comparing the number of antimicrobial patent filings to filings in relation to new anticancer agents (here), which are considered as high-return medicines, it is evident that more encouragement and/or incentivisation for antimicrobial research is required.
The top filers of patent applications relating to antimicrobial agents (2015 – 2024) are shown in Table 1 below.
Table 1: Top 20 patent filers in the field of antimicrobials & the corresponding number of patent family applications
| Optimized Assignee | # Patent Families |
|---|---|
| CHINESE ACADEMY OF SCIENCE | 297 |
| PLA ACADEMY OF MILITARY MEDICAL SCIENCES | 212 |
| SUN YAT-SEN UNIVERSITY (CHINA) | 154 |
| UNIV SOUTHWEST | 114 |
| INST AGRIC RESOURCES & REGIONAL PLANNING | 105 |
| UNIVERSITY SHANDONG | 102 |
| ROCHE HOLDING LTD. | 90 |
| FUDAN UNIVERSITY | 83 |
| UNITED STATES HEALTH & HUMAN SERVICES | 78 |
| SHANGHAI INSTITUTE OF MATERIA MEDICA | 77 |
| GILEAD SCIENCES INC. | 73 |
| JANSSEN PHARMACEUTICA NV | 69 |
| UNIV SECOND MILITARY MEDICAL | 68 |
| MERCK & CO. INC. | 67 |
| CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIC | 65 |
| UNIV SOUTH CHINA AGRIC | 65 |
| VIIV HEALTHCARE LTD | 65 |
| UNIV CHINA AGRICULTURAL | 58 |
| ZHEJIANG UNIVERSITY | 57 |
| INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE | 52 |
Table 1 shows that the top six applicants are from China. This reflects the global trend of patent filings in the last decade, with China-based organisations steadily increasing China's patent filing presence across many areas of technology. Nonetheless, one can identify a few notable pharmaceutical companies, including Roche, Gilead, Janssen, and Merck. It is evident, however, that not all pharmaceutical companies are placing as much importance as the above-indicated companies when it comes to the discovery of new antimicrobials, which is concerning.
A more detailed yearly breakdown of patent families filed since 2015 for each of the above top 20 applicants was conducted.

Although the patent filing activity initially appears to follow no particular general pattern and therefore to be merely dependent on each applicant's own decisions, it does appear that some of those applicants were particularly affected by the Covid-19 pandemic, either resulting in a drop in filings around or since 2020 (e.g. Roche, Shangai Institute of Materia Medica), or conversely in an increase in filings around or since 2020 (e.g. Sun Yat-Sen University, US Health and Human Services, Gilead). This may be due in part to the particular area of expertise of these institutions, which may be either aligned with the research in and around COVID and/or antivirals, or not.
We analysed the individual jurisdictions that were covered by each patent family. The results are presented below in Table 2 and in Figure 2, taking into account the top ten jurisdictions covered.
Table 2: Number of patent family applications published in the field of antimicrobials by country code listed in publication number of INPADOC patent family members
| Family Member | # INPADOC Patent Families |
|---|---|
| CN - China | 8774 |
| WO - PCT | 4643 |
| US United States | 3524 |
| EP - European Patent | 2948 |
| JP - Japan | 2302 |
| CA - Canada | 1835 |
| AU - Australia | 1630 |
| KR - Korea | 1623 |
| BR - Brazil | 1001 |
| MX - Mexico | 914 |
| IL - Israel | 813 |
| TW - Taiwan | 687 |
| ES - Spain | 420 |
| SG - Singapore | 394 |
| AR - Argentina | 329 |
Figure 2: Number of patent family applications published in the field of antimicrobials by country code listed in publication number of INPADOC patent family members

As could be expected from the list of top applicants discussed above, the most prominent jurisdiction is China. Not taking into account the PCT, which only provides a platform for entering specific jurisdictions, China was followed by the USA, Europe, Japan, Canada, Australia, South Korea, Brazil, Mexico, Israel, Taiwan, Spain, Singapore and Argentina.
Also of interest was the country of first filing for each patent family. The results are presented below in Table 3 and in Figure 3 below, taking into account the top ten jurisdictions of first filing.
Table 3: number of patent family applications published in the field of antimicrobials by priority country (i.e. country of first filing)
| Priority Country | # Patent Families |
|---|---|
| CN - China | 6868 |
| WO - PCT | 3272 |
| US - United States | 3035 |
| EP - European Patent | 644 |
| JP - Japan | 460 |
| KR - Korea | 421 |
| GB - United Kingdom | 280 |
| IN - India | 236 |
| AU - Australia | 130 |
| BR - Brazil | 100 |
| IT - Italy | 77 |
| RU - Russia | 61 |
| FR - France | 52 |
| MX - Mexico | 37 |
| DE - Germany | 30 |
Figure 3: number of patent family applications published in the field of Antimicrobials by priority country (i.e. country of first filing)
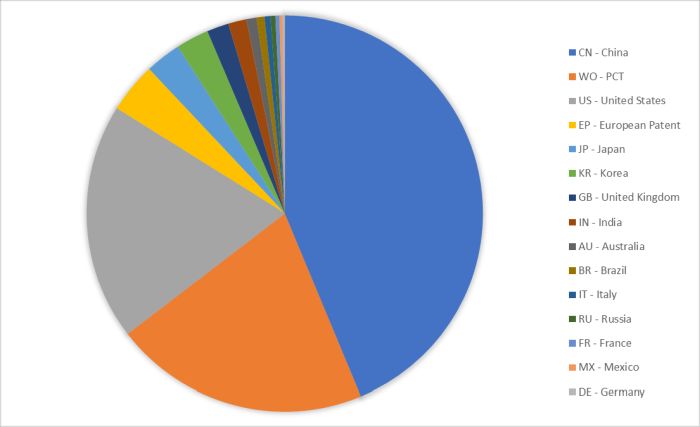
Unsurprisingly, the results strongly overlap the results relating to the jurisdictions covered by each patent family discussed above. Of note was the even greater presence of China in top spot, which is consistent with the names of the top filers discussed above. Also of note is the presence in the Top 10 of India and the United Kingdom, demonstrating the research in that field by organisations based in these two territories. This is despite these countries not being in the Top 10 for the chosen jurisdictions covered by those patent families. A possible explanation is that protection in the UK can be obtained via a European application, and therefore GB may not be pursued separately within a patent family despite being a prominent territory for first filings in that field. The presence of India in this list would suggest high research activity originating from India in this field, but a relatively lesser interest from other filers in seeking protection in that territory. This apparently high level of research originating from India is perhaps not surprising given the recent report from WHO highlighting that India bears the world's highest burden of antibiotic-resistant TB. In 2019 alone, nearly 300,000 people in India died from AMR-related infections.
The initial data simply looked at patent applications directed to antimicrobials in general. However, arguably the greatest concern with regard to AMR is in relation to antibacterial antibiotics and developing resistance. Thus, we wanted to look at the patent filing trends more closely, in order to see what differences may be observed between antibacterial, antiviral and antifungal patent filings.

Figure 4: The number of first filings directed to antibacterials, antifungals, and antivirals, broken down by jurisdiction of first filing. Rest of World includes first filings in the UK, India, Japan, the Republic of Korea, and at the EPO.
Breaking down 'antimicrobials' into antibacterials, antifungals, and antivirals reveals additional trends. Figure 4 shows that antibacterials and antivirals dominate first filings (that is, priority-founding patent applications), with over 2000 families each, compared to around 1500 families directed to antifungal innovation. Examining first-filing jurisdictions highlights where basic R&D occurs. This is often because local patent attorneys tend to favour filing first in the originating country, not least due to security restrictions imposed by many jurisdictions on first filings outside an inventor's home nation. Figure 4 shows that the United States accounts for nearly half of antiviral patent families but only 20–25% of antibacterial and antifungal first filings. Clearly, research entities in China see the development of antibacterials and antifungals as a particular priority, accounting for about a third of first filings in said categories. Whilst it is perhaps not unsurprising that China is the world's largest consumer and producer of antibiotics, its per capita antibiotic use is in fact approximately 10 times higher than in the United States.

Figure 5: The number of destination filings directed to antibacterials, antifungals, and antivirals, broken down by jurisdiction.
Moving on to destination filings – patent applications claiming priority from an earlier application (e.g. a first filing) – we can see in Figure 5 that some trends reappear. Notably, antibacterial and antifungal filings remain significant in China. However, we also observe a new development. That is, whilst the antiviral grouping was only in second place for the number of first filings (Figure 4), it takes the lead in the number of destination filings, with a relatively even spread across the jurisdictions. This may suggest that antiviral technologies more successfully progress through the later stages of clinical development, with such success resulting in a drive for global protection – this is also evidenced by the considerable number of PCT (international) applications filed under the classification of antivirals. It may also be indicative of the types of entities that are filing the patent applications – large pharmaceutical companies generally opt for such broad filing strategies.

Figure 6: The number of patent families assigned to selected entities, broken down by classification. SYSU = Sun Yat-Sen University; AMMS = Academy of Military Medical Sciences; CAS = Chinese Academy of Science; NMU = Naval Medical University. Icons made by Freepik from www.flaticon.com
So which entities are the biggest filers in each of these classifications, and how diverse are their portfolios? In Figure 6, we have selected some of the top assignees in the antimicrobial space, and delineated their filings by the three groupings. US pharmaceutical company Gilead is comfortably at the top, with almost all patent families directed to antivirals. A few Chinese research institutions, namely Sun Yat-sen University (SYSU), The Academy of Military Medical Sciences (AMMS), and the Chinese Academy of Science (CAS), contribute significantly to the large number of antibacterial and antifungal patent applications filed in China, indicating that China's efforts against AMR are well supported by the state. Interestingly, these institutions are also disrupting the once dominant European pharmaceutical companies, with familiar names such as Novartis and Roche falling behind.
Discussion
It is clear that the number of patent filings for antimicrobials remains relatively low, as compared to other medical fields, such as anticancer agents, and this correlates closely with the number of antibacterial agents in clinical and preclinical development. In 2024, the WHO reported (here) that the number of antibacterial agents in clinical development had increased from 80 in 2021 to 97 in 2023. However, compare this to there being over 2000 oncology drugs currently in development (here) and one can readily appreciate the need for increased antimicrobial discovery.
Clearly, more needs to be done to facilitate the development and commercialisation of new antimicrobials, as previously suggested by the O'Neill report of 10 years ago. Very recently, a global health partnership between the Gates Foundation, Novo Nordisk Foundation, and Wellcome was announced (the Gram-Negative Antibiotic Discovery Innovator (Gr-ADI)), to address global health challenges that disproportionately impact people living in low- and middle-income countries. The focus of the initial request for proposals is on the discovery of antibiotics with broad spectrum activity against Enterobacteriaceae, the Gram-negative family of bacteria. Enterobacteriaceae are included on the World Health Organization's list of critical priority pathogens and are among the leading contributors to global AMR-associated deaths, disproportionately impacting people living in low- and middle-income countries, where the drivers of AMR are exacerbated by poverty and inequality.
Other initiatives, such as PACE (Pathways to antimicrobial clinical efficacy), which was set up by Innovate UK and the Flemming Initiative, are looking to encourage antimicrobial development and their commercialisation.
Initiatives such as these are clearly welcomed, but more concerted governmental and collaborative action is required.
Will we see novel sources for identifying new antimicrobials and/or could artificial intelligence (AI) have a role to play?
The vast majority of existing antibiotics come from natural sources and the majority of new antibiotics under development still do. In fact, most antimicrobials come from microorganisms themselves. However, in a recent paper (here), the authors reported that semi-purified hemolymph protein extract from oysters has an antimicrobial effect and can be used to enhance the effectiveness of existing antibiotics. Enhancing the effectiveness of existing antibiotics can be extremely beneficial, as it allows lower doses of antibiotics to be administered and yet still be effective.
AI is being used to develop new antimicrobial compounds, but it may also be used to make hypotheses relating to antimicrobial resistance and open up new ways to target this. For example, it was recently reported (here) how Google's AI "co-scientist" – designed to facilitate researchers by providing its own ideas and theories, was able to suggest in two-days a mechanism by which viruses are able to affect different species, where a team from Imperial College London, through research, took 10 years to elucidate. Although laboratory work would still be required in order to prove any theory, AI may be used to guide the research and reduce the amount of time required to develop new antimicrobials.
Of course, there is great excitement in relation to the use of AI in drug discovery and development. However, there are drawbacks in terms of the data and how it was obtained, being input into AI modeling systems. Also, much of the data that is published is only positive data; in terms of AI learning, negative data can be of significant importance also. Thus, for AI to be of significant use in the discovery and development of new antimicrobials, it may be necessary for there to be a degree of standardisation when it comes to conducting experiments and reporting results, including the reporting of negative results, which may be as important as positive ones.
Could modifying, or better use of existing antibiotics, rather than simply looking to identify new antibiotics, be a possible route forward? In a recent interesting article, Padillia and Norwick (here) reported the efficacy of a vancomycin-texiobactin conjugate molecule that was particularly effective against methicillin-resistant Staphylococcus aureus (MRSA) and even vancomycin-resistant Enterococci (VRE), where vancomycin alone was ineffective. The initial results look promising, but the authors hope that further modifications of the conjugate molecules may lead to further improvements in efficacy.
In separate work (here), an international team led by a team based in Melbourne has also been studying the use of rifaximin to treat liver disease and found that its use is leading to an acceleration of resistance to daptomycin - one of the remaining effective antibiotics against VRE infections. It was unexpected that rifaximin would result in such resistance developing and the team showed that this was due to genetic alterations in RNA polymerase within bacteria, which could potentially make them resistant to other antibiotics and not just daptomycin. The work points to the care that needs to be exercised when administering antibiotics and also to the significant importance of concurrent rapid susceptibility testing when looking to use antibiotics to treat infections.
It may also be possible to enhance the effectiveness of existing antibiotics, by better understanding how antimicrobial resistance mechanisms develop in bacteria. Recently, Rahman et al. (here) created a collection of bacterial mutants using CRISPR interference (called CIMPLE – CRISPRi-mediated pooled library of essential genes), to help them understand how a new type of antimicrobial molecule, discovered by AI, stops bacteria from growing.
Also, identifying new targets can be important in the drug discovery process. Recently, a team of scientists led by researchers from the John Innes Centre in the UK published an article (here) looking at how certain molecules shut down bacterial expression in a multi-drug-resistant plasmid in bacteria and how this leads to the plasmid surviving. Understanding how bacteria evade antimicrobial action, such as by developing resistance, can facilitate researchers in their goal of identifying new antimicrobials that may be less susceptible to resistance development.
Finally, methods of treating or preventing microbial infections, other than by using antimicrobial compounds, may be considered. The development of vaccines is an obvious consideration, particularly in light of the success in managing the global Covid pandemic. In a future article, we will take a look at patent filing statistics relating to antimicrobial vaccines and see what conclusions may be drawn from this.
Alternatively, bacteriophages could be used to treat bacterial infections. Bacteriophages, or phages, are viruses that can infect and inhibit or kill bacteria. Bacteriophages have been used in Georgia and other former Soviet Union states for decades, but have not generally received recognition or approval for use in most other countries. Could the use of bacteriophages be an alternative or complement to the use of antibiotics? Perhaps, but bacteria have their own immune systems that protect against phage attack. In a recent study "AcrIIIA1 is a protein–RNA anti-CRISPR complex that targets core Cas and accessory nucleases", Chou-Zeng et al. (here), identified the protein AcrIIIA1 within some phages is able to overcome the CRISPR-Cas defence mechanism found in bacteria and so the use of phages to treat bacterial infections also comes with associated difficulties.
Clearly, there is a great deal of scientific interest in the AMR field, as evidenced by the publications and initiatives being put forward. However, our patent analysis still shows that much more needs to be done in terms of converting this interest into the increased filing of new patents and the potential commercialisation of new antimicrobials. Hopefully, we will see an increase in new patents more quickly coming through, but the concern surrounding increased resistance developing through the use of existing antibiotics means that this cannot come soon enough.
"If we don't contain AMR and we don't get new antibiotics, we lose modern medicine. More people will die of infection than of cancer." Professor Dame Sally Davies, UK Special Envoy on AMR
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.





