China's Supreme People's Court recently handed down an influential decision on the validity of a Chinese pharma patent covering Takeda Pharmaceutical's star product alogliptin (alogliptin case) - a ruling that contains important guidance on the standard for testing what is the same subject matter for the purposes of priority examination. Selected as one of the SPC's biggest cases of 2023, the decision will be applied in the priority examination during all future patent invalidation proceedings and administrative litigation.
In China, the proper standard for this has not been very clear or consistent in practice. The China National Intellectual Property Administration applies a "substantially the same" standard, which is also used in novelty assessment. This involves examining whether the claimed solution as a whole in the later patent has the same technical field, technical problem to be solved, technical solution and expected effect as the earlier application ((2021) SPC no. 593 Judgment).
In contrast, the courts have tended to use a more rigid "directly and unambiguously derived" standard, which requires every feature in claims of later patents to be recited in the earlier application.
In previous decisions, the SPC had never carefully differentiated the two standards because in most cases both standards lead to the same conclusion in priority examination. However, in the alogliptin case the situation is different because the claims include administration features.
The "substantially the same" standard would ignore the administration features if these features cannot distinguish the claimed solution in the later patent from the earlier application. But the subject matter is not the same between the later and earlier patents since the administration features are not recited in the earlier patent according to "directly and unambiguously derived" standard.
In the new ruling, the SPC for the first time clarifies that the standard for testing the same subject matter in priority examination should be "directly and unambiguously derived" standard rather than the "substantially the same" standard.
Key points of alogliptin case
The basic facts of the alogliptin case are not complicated and mainly involve the examination of priority. The case details are shown in Figure 1. There are two main issues in dispute:
- what criteria should be applied to determine the "same subject matter" in the priority examination; and
- whether features that did not put limitation on the scope of claims should be considered in the examination of the same subject matter of priority.
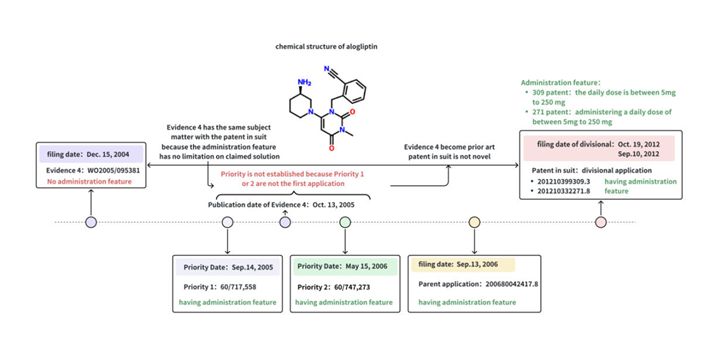
Figure 1: Schematic diagram of the Alogliptin case
The SPC ruled that the "directly and unambiguously derived" standard should be applied in the examination of the "same subject matter" of priority. The SPC opined:
- From the perspective of legislation, the foreign priority system stipulated in the first paragraph of Article 29 of the Patent Law is to ensure that the applicant does not lose the benefit of the first application due to the difference in the application time between different countries and regions. The application of the "directly and unambiguously derived" standard in the examination of the same subject matter is consistent with the legislative purpose of the priority system.
- The substantive limitations of some features on claimed solution could be considered from the patentability side when analysing novelty and inventive But the examination of priority aims to confirm whether the filing date of the prior application can be used as a priority date, and should not involve the patentability considerations into priority examination.
- Priority examination should be the previous step before examining novelty and inventive In this step, it should apply the standard for priority examination rather than involving standard used in novelty and inventive step examination.
- If using the standard of substantive limitation on claimed solution during the examination of priority, the priority will be well-established as long as the patentee adds features into the claim that have no limitation on examination of novelty and inventive This is inconsistent with the purpose of the priority system and will also make the patentee obtain improper benefits.
- According to the Guidelines for Patent Examination, the standard for determining the same subject matter should be the "directly and unambiguously derived" standard, which is different from the "substantially the same" standard for novelty examination.
Not to be confused with "the same invention" for novelty purposes
Although the Chinese Guidelines for Patent Examination provide rules and doctrines for testing the same subject matter of priority, there is no clear guidance on the relation between "the same subject matter" in priority and "the same invention" in novelty assessment. Similar wording used in the Guidelines makes the boundary between standards applied in priority and novelty examination even more vague. Specifically:
- Section 1.2 of Chapter 3 of Part II gives the definition of "the same subject matter" in priority: An invention or utility model with the same subject matter as mentioned in Article 29 of the Patent Law refers to an invention or utility model relating to the same technical field, solving the same technical problem, having the same technical solution and achieving the same expected effect.
- Section 1 of Chapter 3 of Part II prescribes the standard for "the same invention" of novelty over prior art: the claimed invention constitutes the same invention over prior art if both of them has substantially the same technical field, the technical problem to be solved, the technical solution and the expected effect.
The CNIPA is inclined to apply the similar standard used in novelty evaluation when examining priority in invalidation proceedings, that is, considering whether the later patent has the same technical field, the technical problem to be solved, the technical solution and the expected effect as the earlier application.
In the alogliptin case, the SPC expressively clarifies, for the first time, that testing the same subject matter in priority examination should mean applying the "directly and unambiguously derived" standard, and should not confuse this with the standard for novelty assessment.
Further, the SPC indicates that according to the essence of establishing priority system, the "same subject matter" in the priority examination underlines the claimed features or solutions in later patent should recite in the earlier application, rather than analysing whether the claimed solutions are substantively the same as prior art. Therefore, the testing standard for priority examination should be different from the standard applied in novelty assessment and it is not appropriate to introduce elements in novelty assessment into priority examination.
Every feature in claims influence priority establishment
After this ruling, the standard for priority examination will be unified to "directly and unambiguously derived" standard in later patent invalidation and administrative litigations.
Under such a standard, every feature of claims in later patent applications will be examined whether they are literally recited or can be ambiguously derived from the earlier application. As for a pharma patent, any features defining medical use, characterisation data, administration methods or manufacturing process should be considered in priority examination regardless of whether those features place limitations on the claimed subject matter or not.
For example, the medical use feature would be considered in priority examination no matter whether this feature has limitation on the structure of claimed compound. If the product claims of the later application include a new medical use feature that are not recited in the earlier application, even though the medical use has no limitation on the structure of the compound, the later application cannot enjoy the priority according to "directly and unambiguously derived" standard.
A new 'Achilles' heel' for patents in China?
The consistency between the earlier and later applications is vital for establishing valid priority. Although it is not required that the expression of the later patent is completely the same as the earlier application, less consistency brings more risk to successful establishment of priority.
Patent holders can try the following things to reduce the risk of losing priority:
- Avoiding using different technical terms in earlier and later applications. If the later patent application uses a technical term that does not have the same meaning or scope of the term recited in the earlier application, the later patent cannot enjoy the priority date of earlier patent. For example, in InterDigital v Huawei, "CSI request field" of claims 1-10 of later patent is not equivalent to "CQI request field" recited in priority document, so that claims 1-10 cannot enjoy the priority date and are all invalidated due to lack of inventiveness over a 3GPP technical standard published between priority date and filing date of the patent.
- Claiming multiple priorities if the later application includes more than one set of Patentees are allowed to claim multiple priorities based on different earlier patent applications. However, it should be noted that the minimum element for claiming priority is one single technical solution, rather than technical features. That is, the claims in later patent restructuring features recited in two or more priority documents are unable to enjoy any priority date of earlier patent applications.
- Avoiding broadening the scope of claims in later patent by When an earlier patent application only recites one specific embodiment of the product. the later patent cannot claim a general product covering different embodiments even if they have the same function and effects. For instance, a hair removal device claimed in a later patent cannot enjoy the priority of a laser hair removal device because the later patent broadens its scope to non laser hair removal devices which are not recited in earlier priority documents ((2023) SPC no. 689 Judgment).
Originally published by IAM, May 2024
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
We operate a free-to-view policy, asking only that you register in order to read all of our content. Please login or register to view the rest of this article.


