Abstract: Combination drug therapy offers significant advantages in treating various diseases and has become important in drug development. With the rapid growth of China's innovative drug industry, the need to protect innovative achievements is increasingly urgent. However, there remains an unmet need for patent protection of combination drug therapy in China. This paper examines the industry background and protection value of combination drug therapy, current judicial practices in China, and patent protection experiences in Europe and the United States. It clarifies the necessity and current limitations of patent protection for combination drug therapy and provides suggestions for improvement.
Keywords: Combination drug therapy, Patent protection, Medicinal use
Combination drug therapy involves the simultaneous or sequential use of two or more drugs to achieve therapeutic goals, aiming to enhance efficacy or reduce side effects.1 It is commonly used to alleviate disease conditions and improve therapeutic outcomes and is also the preferred method for treating complex diseases. As such, it has become important in drug development. Currently, there are three main forms of combination drug therapy:
- Fix-Dosed Combination Finished Pharmaceutical Products (FDC-FPPs): Creating FDC-FPPs from two ctive ingredients, such as BI Company's diabetes drug linagliptin-metformin tablets.
- Packaged Combinations: Packaging two different active ingredient preparations in the same kit or combination product, such as Pfizer's COVID-19 drug nirmatrelvir/ritonavir tablets.
- Simple Drug Combinations: Listing combination regimens in drug label, such as Junshi Biosciences' toripalimab indicated for use with cisplatin/gemcitabine as a first-line treatment for adult patients with metastatic or recurrent locally advanced nasopharyngeal carcinoma. These drugs often cannot be made into a FDC-FPP due to the their nature or is unsuitable for development into a combination product due to different ownership.
For the first two forms, current product patents (such as composition patents and kit patents) and medicinal use patents can provide protection. However, the patent protection for the third form still faces many challenges and unmet needs in China. This paper focuses mainly on the third form of combination drug therapy. By analyzing the industry background and protection value of combination drug therapy, current judicial practices in China, and the patent protection experiences in Europe and the United States, this paper clarifies the necessity and current limitations of patent protection for combination drug therapy and provides suggestions for improvement.
I. Industry Background of Combination Drug Therapy
1. Combination Drug Therapy as an Innovative Technical Solution
Article 27 of the "Drug Registration Administration Measures" stipulates that "if a drug approved for clinical trials intends to add indications (or functions) or to combine with other drugs, the applicant shall submit a new drug clinical trial application, which can only be carried out after approval." This means that combination drug therapy has the same review and approval requirements as indications. In practice, combination drug therapy is also listed under the "Indications" section as part of drug indications. From the perspective of drug approval, the development of combination drug therapy is similar to that of indications. Therefore, like new indications, combination drug therapy is also an innovative technical solution. It is obtained by pharmaceutical companies through extensive drug research and development activities, validated through clinical trials and approved by the National Medical Products Administration (NMPA), aimed at solving specific technical problems (such as treating specific diseases or alleviating specific symptoms). Thus, combination drug therapy has a foundation for patent protection.
2. Unique Therapeutic Advantages of Combination Drug Therapy
Numerous clinical data indicate that combination drug therapy has significant advantages over monotherapy in treating various diseases. For example, in cancer treatment, the combination of ramucirumab and paclitaxel has shown better efficacy than paclitaxel alone in treating advanced gastric cancer.2 In the field of hypertension treatment, most patients choose combination drug therapy from the initial treatment stage, which has become a trend in treating hypertension. In China, more than two-thirds of hypertension patients need to take two or more medications to effectively control blood pressure. For infections with multidrug-resistant pathogens, combination drug therapy may provide a broader antimicrobial spectrum, synergistic effects, and reduced resistance, making it an important direction in treating multidrug-resistant infections.
It is evident that compared to monotherapy, combination drug therapy shows superior therapeutic effects in treating certain diseases, significantly alleviating patient suffering. Therefore, combination drug therapy is an innovative technical solution that can bring beneficial technical effects. Patent protection for combination drug therapy aligns with the legislative purpose of encouraging invention and creation, promoting scientific and technological progress, and fostering social development.
3. Focus of Chinese Pharmaceutical Companies on Combination Drug Therapy
Recent research results indicate that combination drug therapy has become a crucial direction in current drug development, with the proportion of clinical research on combination drug therapy continually rising, far exceeding that of monotherapy. A study by the University of Macau on the clinical research of 72 oncology drugs approved from 2017 to 2021 shows that the proportion of research on monotherapy is decreasing year by year. By 2021, the proportion of monotherapy research was only 20-30% (blue line).3
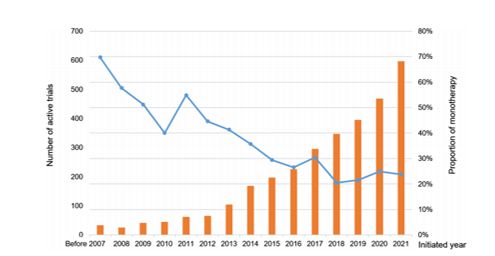
Figure 1. Number of clinical trials initiated annually and proportion of monotherapy for 72 oncology drugs approved between 2017-2021 (The bar chart shows the number of new clinical trials initiated each year, and the blue line represents the proportion of monotherapy clinical trials for the same period).
In this trend, the cooperative development of combination drug therapies to achieve a win-win situation has become an inevitable choice. This collaborative model not only expands the applicable fields of the drugs but, more importantly, brings more and more effective medication options to patients, improves their quality of life, and promotes harmonious social development.
At the same time, combination drug therapies have also become an important research and development direction for Chinese innovative pharmaceutical companies. Taking the clinical pipeline of the Chinese innovative drug company Inventis Bio as an example (see the figure below), more than half of the company's clinical pipeline involves combination drug therapies, with multiple clinical pipelines involving collaborative development with multinational pharmaceutical companies such as Pfizer and Merck. This is not an isolated case for Chinese innovative pharmaceutical companies. In addition to Inventis Bio, other innovative pharmaceutical companies such as Akeso,4 inxmed,5 and Hutchison China MediTech6 have clinical pipelines with over 60% involving combination drug therapies.
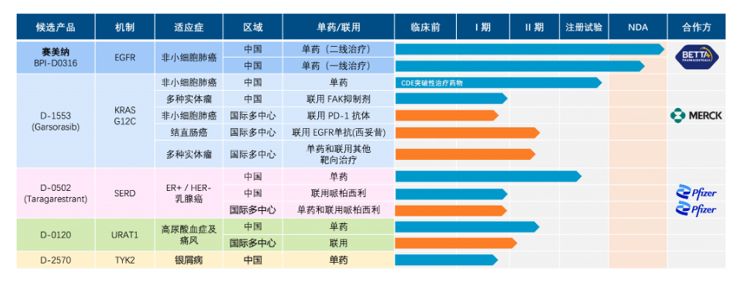
Figure 2. Overview of Inventis Biotherapeutics Product Pipeline7
Therefore, implementing patent protection for combination drug therapies not only aligns with the trend of technological innovation in the pharmaceutical field but also meets the inherent need for Chinese pharmaceutical companies to protect their innovative achievements. This aligns with the national policy of encouraging pharmaceutical innovation.
In conclusion, granting patent protection that matches the innovative contributions of combination drug therapies not only conforms to the current trend in pharmaceutical research and development and has a feasible legal basis but also aligns with the legislative spirit of patent law and the national policy of encouraging pharmaceutical innovation.
II. Judicial Practice and Challenges in China
Combination drug therapy technical solutions typically involve developing new uses for known active ingredients. For combination drugs that are not suitable for development into FDC-FPPs or combination products, China usually protects them through medical use patents.
The essence of medical use patents is using known products to treat new indications, which are disease treatment methods. However, from a humanitarian perspective, China upholds the principle that doctors should be free to choose various methods and conditions during diagnosis and treatment. Therefore, to avoid impacting doctors' diagnostic and treatment processes, China initially did not protect medical use technical solutions. With societal development, China gradually recognized that new medical use solutions for known substances contribute to innovation. Since 1993, China has begun granting patent rights for medical uses. The "Patent Examination Guidelines (1993)" regulated the same claim format for medical use as the so-called Swiss-type claims used by the European Patent Office, i.e., protecting the medical use in the form of "use of compound X for the preparation of a medicament for treating disease Y,"8 which is still used now. From a legislative technical perspective, this drafting method avoids granting patents for treatment methods while affirming and protecting new uses of drugs, encouraging innovation to some extent. However, due to China's legislation and judicial practice imposing strict limitations on the protection scope of such patents, this form of protection has gradually become misaligned with the development of innovative drugs, restricting the protection of pharmaceutical innovation in China, especially for combination drug therapies.
1. Current Interpretation of the Protection Scope of Medical Use Patents in China
The "Patent Examination Guidelines 2010"9 explicitly stipulate that claims of the medical use of substances belong to pharmaceutical processes. It also stipulates that when considering the "novelty of the use of chemical products," whether the features relating to use, such as the target of administration, the method of administration, the route, dosage, and time interval, have limitation functions on the pharmaceutical process should be considered. Differences merely in the medication process cannot render the use novel. This provision has remained unchanged in subsequent revisions of the "Patent Examination Guidelines." How to interpret the "pharmaceutical process" directly determines the scope of the protectable technical solutions and how to interpret the protection scope of such patents.
Objectively, drugs, as special commodities, need to undergo strict administrative approval before marketing. Therefore, if interpreting the pharmaceutical process broadly, all activities conducted by pharmaceutical companies to ultimately bring active substances to market should be considered part of the pharmaceutical process. The activities include the research and development in dosage, methods of administration, and combination of drugs following the requirements of both the Drug Administration Law and the regulatory approval by the drug administration, and the formulation of drug labels, as these activities are essential to produce marketable drugs. However, if a narrow interpretation is adopted, the pharmaceutical process can be understood as the physical preparation process of the drug. The technical features, such as dosage and administration intervals, would belong to disease treatment methods rather than the pharmaceutical process.
In current judicial practice, Chinese judicial authorities strictly interpret the "pharmaceutical process" in a narrow way. For example, in the Cubist Pharmaceuticals10 and Genentech11 cases, the Supreme People's Court clarified that the "pharmaceutical process in the sense of patent law generally refers to the behavior of preparing specific drugs themselves using specific steps, processes, conditions, raw materials, etc.," and that "what usually has a direct limiting function on it are raw materials, preparation steps and process conditions, drug product forms or components, and equipment. For features that only involve drug use methods, such as dosage and time intervals, if these features do not have a direct relationship with the pharmaceutical processes, they essentially belong to the specific methods of using the drug on the human body after obtaining the drug through the pharmaceutical processes, which do not have a direct and inevitable relationship with the pharmaceutical processes. Such features manifested only in medication behavior are not technical features of pharmaceutical use and do not limit the pharmaceutical process claimed in the patent." The Supreme People's Court has consistently interpreted the "pharmaceutical process" as the physical preparation process of drugs, and features such as the target of administration, method of administration, use, and dosage do not limit such claims and cannot be protected through such claims. The Supreme People's Court has consistently upheld this interpretation through typical cases.
In this way, during the prosecution and invalidation stage, patent rights are typically determined based solely on the characteristics of the target indication and pharmaceutical method. In the enforcement stage, the protection scope of medical use patents is interpreted as the preparation method of the drug product for treating specific diseases.
2. Limitations of Patent Protection for Medical Uses
As the field of drug research and development continues to evolve, the current interpretation of patents for medical use has increasingly shown its limitations. It fails to equally protect the contributions of pharmaceutical innovations, which is particularly evident in the protection of combination drug therapy.
If the claims for combination drug therapy are written as "use of compound X for the preparation of a medicament for treating disease Y, wherein X is used in combination with Z," the feature "X is used in combination with Z" will not be considered in determining novelty and inventiveness since it does not influence the pharmaceutical process. If "use of compound X for the preparation of a medicament for treating disease Y" itself does not demonstrate the innovation, it may not be granted due to a lack of novelty and inventiveness.
Another way to write the claims is "use of A and B in the preparation of a combination for treating disease C." However, authorities tend to interpret this as the preparation of FDC-FPPs (or drug packages) from A and B, which does not cover drug combination therapy. For example, in the invalidation case12 of the drug combination therapy of Antibody A and Chemical B in 2023, the claim was written as "use of Antibody A and Chemical B in the preparation of a combination for treating disease C." In the decision, the China National Intellectual Property Administration (CNIPA) stated, "for the type of invention that involves 'drug combination therapy,' since such inventions can typically be implemented by preparing FDC-FPPs or a drug package or kit combining the active components of each drug, the features of 'drug combination therapy' in the technical solutions should involve the raw materials and pharmaceutical process, and thus should be recognized as having substantial limiting functions on the pharmaceutical method claimed in the patent."
Notably, in interpreting the claims, the invalidation decision, without internal or external evidence, aimed to maintain the validity of the patent by defining the claims as "the use of Antibody A and Chemical B in the preparation of a drug package product for treating disease C." Meanwhile, the core reason for maintaining the validity of the claims was the technical effect obtained from "drug combination therapy." In essence, this effect is derived from the method of drug combination, not from overcoming technical difficulties to prepare a "drug package" or "kit." This interpretation approach is logically inconsistent. In fact, the invalidation decision positively acknowledged that the method of "combination therapy" offers a beneficial technical effect in treating disease C, thus acknowledging its novelty and inventiveness. However, constrained by the current narrow interpretation framework of the "pharmaceutical process," to maintain patent validity, the method of "combination therapy" was forced into the form of preparing a "drug package or kit," even though the patent specification did not describe a "drug package." This situation reflects the contradiction between the current interpretation framework of China's patent protection for medical uses and the essence of the invention. This contradiction leads to the current limitation, where the contributions of pharmaceutical innovations cannot be equally protected, particularly in enforcing combination drug therapy patents.
In the related patent linkage case of the invalidation case,13 the Supreme People's Court endorsed the CNIPA's interpretation method for such claims. The Supreme People's Court stated that there could be two interpretations for such claims: one is the active substances form FDC-FPPs, and another is two active substances form a drug combination product through specific packaging forms, such as a drug package or kit. The Supreme People's Court further clarified that, according to the above interpretation, only if use claims conform to the type of pharmaceutical method, such claims can be granted. If interpreted as combination use, such combination use would belong to methods of diagnosing and treating diseases, which cannot be granted. Therefore, the Supreme People's Court adhered to the narrow interpretation framework of the "pharmaceutical process" when interpreting such drug combination claims. Consequently, even though the label of the involved drug described the accused combination therapy, the Supreme People's Court determined that it did not fall within the protection scope of the involved patent.
However, the current narrow interpretation framework of the "pharmaceutical process" does not meet the needs of pharmaceutical companies to protect the innovative outcomes of combination drug therapies. On one hand, combination therapy solutions have become an essential part of pharmaceutical innovation. The related clinical trials are far exceeding those of single-drug trials, leading to an increasing demand for patent protection for combination therapies. On the other hand, the collaborative development of combination therapies by different companies has become a mainstream trend.
During the development of combination therapies, if it is feasible to prepare FDC-FPP or "drug package," developers often prefer to protect it through product claims. The importance of medical use patents is particularly highlighted for products unsuitable for development into FDC-FPPs or "drug packages."
From the aforementioned invalidation decisions and court judgments, it is evident that the CNIPA has positively acknowledged the technical contributions of "combination therapy" itself. However, the interpretations of "drug combinations" by the CNIPA and the Supreme Court do not align with the actual contributions of the technology, resulting in the claims being maintained but unusable in enforcement, effectively nullifying patent protection. This handling approach cannot effectively support China's national policy of deepening pharmaceutical innovation reform.
3. Beneficial Exploration by Chinese Judicial Authorities
In response to the challenges in patent protection for combination drug therapies, Chinese judicial authorities have made some beneficial explorations. In the first-instance judgment of the aforementioned related patent linkage case,14 the Beijing Intellectual Property Court reverted to the use nature of such claims when interpreting them.
Specifically, the Beijing Intellectual Property Court held that the claimed use relates to the combination use and specific dosage of Antibody A and Chemical B. Whether they are packaged into one product does not affect their combination use or specific dosage, nor does it impact the claimed use. The involved claims not only specify the combination use of Antibody A and Chemical B but also individually define their dosages. Under these circumstances, a person skilled in the art would typically understand that Antibody A and Chemical B are administered in specific doses and used together to treat a certain disease.
Unfortunately, this judgment was eventually overturned by the Supreme People's Court. However, the Beijing Intellectual Property Court's decision made significant explorations in returning the "Swiss-type claims" to their essential use nature, detaching them from the physical pharmaceutical process framework.
III. Experiences from Europe and the United States on Combination Drug Therapy Protection
The innovative drug industry is highly intellectual property-intensive. Given the significant R&D cost gap between branded and generic drugs, losing strong patent protection for branded drugs can deal an irreparable blow to their development, thus harming drug innovation and human quality of life. Therefore, many leading countries and regions in pharmaceutical innovation provide comprehensive protection for drug-related patents, including those for combination drug therapies. The following section provides an overview of the experiences of Europe and the United States in protecting combination drug therapies, to offer references for the current issues in China's medical use patent protection.
1. European Practice on Medical Use Patents
For combination drug therapies that are not FDC-FPPs or drug package products, Europe also adopts the protection mode of medical use patents. Initially, Europe used Swiss-type claims to protect medical use, later shifting to purpose-related product claims.
Similar to China, the European Patent Convention (1973)15 and the European Patent Convention (2000)16 stipulate that methods of treatment and diagnosis for humans and animals cannot be patented.17 Additionally, the European Patent Convention (1973) specified the first medical use: if a substance is known but its use as a medicine is not disclosed, the product for medical use can be patented. However, other medical uses of known substances (the second medical use) were not recognized for their novelty.
In 1984, the Swiss Patent Office first accepted the claim "use of substance X for the preparation of a medicament for diagnosing or treating disease Y." Subsequently, in the G5/83 case, the Enlarged Board of Appeal of the European Patent Office (EPO) accepted this kind of claim, leading to the widespread use of Swiss-type claims to avoid the prohibition on methods of treatment and diagnosis.
The patentability of Swiss-type claims has been contentious, especially regarding whether features like dosage can impart novelty to the claims. Early cases such as T317/95, T56/97, and T4/98 generally held that dosage features were typical of medical practice and could not impart novelty or inventiveness. However, the Genentech case18 recognized that improvements in dosage could bring novelty to Swiss-type claims, influencing the examination of similar cases, and gradually unifying the standards.
The European Patent Convention (2000) in Article 54(5)19 further established the patentability of the second medical use of substances. According to the EPC (2000), Swiss-type claims can still be used to protect the second medical use of substances.
Until 2010, the Enlarged Board of Appeal in the G2/08 decision pointed out that the second medical use of substances should be drafted as purpose-related product claims, i.e., "Substance X for use in the treatment of Y," rather than Swiss-type claims. Although both formats include the features of the substance and its use, Swiss-type claims additionally include manufacturing features. However, according to EPC (2000) Article 54(5), the second medical use does not need to include these features, distinguishing the two drafting formats.
Moreover, the Enlarged Board of Appeal concluded that when protecting the medical use of substances under EPC (2000) Article 54(5), the subject matter of the claim is a product, but its novelty and inventiveness derive from its medical use. This special provision of the patent system does not distinguish between a disease and a dosage for the drug. Therefore, when the dosage is the only feature not disclosed by prior art, EPC (2000) Article 54(5) does not exclude its patentability, and there is no reason to treat indications and dosages differently.
Currently, under EPC (2000) Article 54(5), purpose-related product claims are used to draft such claims, freeing them from the constraints of the pharmaceutical process. Claims for the second medical use of substances can protect known drugs for new diseases and new treatments for known diseases, including new indications, administration routes, subjects, and dosages, providing novelty and inventiveness to the claims. This provision offers a legal basis for patent protection of combination drug therapies.
Notably, even with Swiss-type claims, combination drug therapy can be protected. For example, in the appeal decision of case T1243/12, the EPO Board of Appeal confirmed the protection of combination drug therapy by Swiss-type claims. The involved claims concerned the use of Rituximab (RTX) in the preparation of a medicament for treating rheumatoid arthritis, where the medicament is used in combination with Methotrexate (MTX). The Board of Appeal determined that the protection scope of the claims included RTX alone, which is combined with MTX for treating rheumatoid arthritis, as well as product combination of RTX and MTX for this purpose. Thus, the EPO Board of Appeal recognized the protection of combination drug therapy by Swiss-type claims.
Additionally, in the Eli Lilly v. Actavis20 case, although the focus was on the application of the doctrine of equivalents, it also reflected the protection of combination drug therapies. The involved patent was granted before 2010 and protected the combination of Imipenem and Vitamin B12 using Swiss-type claims. Even though the defendant only mentioned the combination in the label and did not prepare a drug package, the court and both parties did not dispute that the combination drug therapy fell within the patent protection scope. The final infringement ruling indicated that the court recognized the Swiss-type claims' protection of combination drug therapies, not requiring the final product to be a kit or combination product containing both substances.
With the G2/08 decision in effect, the EPO uses purpose-related product claims instead of Swiss-type claims, which align the second medical use with its essential use and eliminate redundant pharmaceutical process constraints on medical use claims, clearly defining their protection scope.
The development of medical use protection in Europe shows that whether using Swiss-type claims or purpose-related product claims, the impact on the pharmaceutical process is not considered, thus returning such claims to their essential use, enabling the protection of combination drug therapies.
2. U.S. Practice on Medical Use Patents
In the United States, combination drug therapies are typically protected as treatment methods. 35U.S. Code §101 stipulates that whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefor, subject to the conditions and requirements of this title. This provision establishes the patentability of disease treatment methods. To avoid restrictions on medical practitioners, 35 U.S. Code § 287 (c)exempts medical practitioner from liability for patent infringement when performing medical activities. 35 U.S. Code § 271 (b) regulates the behavior of generic drug companies through inducing infringement.
Under the U.S. framework, there are no obstacles to patenting combination drug therapies as treatment methods in the claims. Similar to the practice in China, enforcement typically relies on the information in the generic drug's label. If the label describes the combination therapy, it can be determined that the generic drug company has induced medical practitioner to implement the infringing behavior of combination therapy. The medical practitioner' exemption system ensures their freedom to practice any treatment method. Under this system, combination drug therapies can be fully protected in the U.S.
A typical case illustrating this is the cabazitaxel case.21 Sanofi is the marketing authorization holder for JEVTANA®, which contains cabazitaxel as its active ingredient. The approved use in JEVTANA®'s New Drug Application (NDA) is for combination use with prednisone to treat hormone-refractory metastatic prostate cancer in patients previously treated with a docetaxel-containing regimen. Sanofi also holds a method patent (US10716777, the '777 patent) protecting the use of cabazitaxel in combination with prednisone to treat metastatic castration-resistant prostate cancer (mCRPC). This patent is listed in the FDA's Orange Book. In 2022, Sandoz submitted an NDA for cabazitaxel, indicating that it was for use as a microtubule inhibitor injection in combination with prednisone for treating mCRPC patients previously treated with docetaxel. Sanofi sued Sandoz for infringing the '777 patent based on this submission. The court analyzed Sandoz's product label and referenced experimental data, concluding that Sandoz's product label would encourage, promote, and recommend the use of cabazitaxel in a way that infringed the 777 patent, thereby confirming the infringement.
Another notable case is the Prandin® (repaglinide) case.22 Although this case primarily involved changing the use code of the patent, Caraco, a generic drug company, sought to remove the indication for "combination use of repaglinide and metformin" to avoid infringing the combination therapy patent of the branded drug.
These cases show that the U.S. patent practice, through the patentability of treatment methods, medical practitioner' exemption, and inducement of infringement, can adequately protect combination drug therapies.
In summary, both European and U.S. practices can provide comprehensive patent protection for combination drug therapies. However, implementing U.S. practices in China would require extensive modifications to the patent system, including the patenting scope and methods of determining infringement, making it a challenging endeavor.
Given that China's patent law was initially formulated with reference to European patent law, and considering the legal system similarities, China can draw on European practices to protect combination drug therapies. From the European Patent Office's (EPO) examination changes, it is evident that initially, Swiss-type claims were interpreted as impacting the pharmaceutical process. However, after the 2004 Genentech case (T1020/03), the EPO gradually reversed this view. The 2010 G02/08 decision further eliminated additional restrictions of the "pharmaceutical process," providing substantial protection for medical use without impacting doctors' freedom to practice.
IV. Suggestions for Improving Patent Protection for Combination Drug Therapies in China
Given the challenges in patent protection for combination drug therapies in China, several approaches can be considered to improve the situation:
1. Adopting Purpose-Related Product Claims
In China, the main issue limiting the protection of combination drug therapies is the overemphasis on pharmaceutical process features in Swiss-type claims, neglecting their essential use features. Swiss-type claims were originally a workaround to protect medical use inventions while formally complying with legal requirements. With legal changes, the EPO has abandoned this format in favor of purpose-related product claims directly reflecting the essence of the invention.
Therefore, to robustly protect medical use inventions in China, adopting the European approach and abandoning Swiss-type claims in favor of purpose-related product claims ("substance A for use in the treatment of disease B") can help. This would allow such inventions to return to their essential use without being constrained by additional "pharmaceutical process" requirements. Although Chinese judicial practice currently does not consider the use feature as limiting the product's structure and composition, resulting in a lack of novelty for such claims, legal provisions can be established to clarify the protection scope for claims specifically directed to certain uses.
Since China's patent law shares many similarities with European patent law, adopting Europe's second medical use writing format would not conflict with China's legal system. This approach aligns with current innovation practices and resolves the constraints of the pharmaceutical process on such technical solutions. In infringement determination, similar to current judicial practice, whether a manufacturer infringes will still rely on the content recorded in the drug's label, allowing integration with current judicial practice.
2. Optimizing the Interpretation of "Pharmaceutical Process"
Without legislative changes, it is recommended to delete the provision in Section 5.4 (4) of Chapter 10 of Part II of the "Patent Examination Guidelines (2023)" and reinterpret "pharmaceutical process" in a broad manner.
Given the drug's need for approval as a special commodity, the broader pharmaceutical process includes all activities required to develop an active ingredient into a drug, such as determining dosage, administration methods, and combination drug use. This broad interpretation aligns with the substantial research and clinical trials required for drug development and would protect inventive dosages, administration methods, and combination drug uses, consistent with the legislative spirit of the patent law and national policies encouraging pharmaceutical innovation.
This interpretation would not restrict doctors' use of drugs since the claim still targets the pharmaceutical process, regulating manufacturers' behavior without imposing additional restrictions on medical practice.
Such an interpretation would not conflict with the existing examination system. Although Swiss-type claims in the medical use of substances focus on "preparing a certain drug," the novelty and inventiveness often derive from the treatment objective, not the preparation method. Therefore, it is reasonable to consider the "combination drug use" feature when assessing novelty and inventiveness.
3. Interpreting "Drug Combination" Reasonably
Given the fundamental nature of combination drug inventions in drug combinations, to meet formal requirements, Swiss-type claims can define " use of drug combination A and B in the preparation of a drug for treating disease C." By reasonably interpreting "drug combination," combination drug use can be protected.
At the prosecution and invalidation stage, a "drug combination" formed by two or more active ingredients can be interpreted as including physically isolated components if they cannot achieve the beneficial effect without each other. This interpretation distinguishes the invention from prior art, allowing patent granted if other regulations are met.
At the enforcement stage, "drug combination" should be similarly interpreted so that whether the ingredients are packaged together does not affect their combined use. If the generic drug label describes combination therapy, it can be considered direct infringement.
4. Protecting Combination Drug Use based on Joint Infringement
Swiss-type claims are purpose-limited method claims. As a fallback solution, the combination drug inventions can be protected based on joint infringement. If Swiss-type claims are understood as "pharmaceutical methods," infringement determination can refer to the joint implementation rules common in the telecommunications field. If different entities implement steps of the pharmaceutical method separately, as long as a single-drug manufacturer provides combination use guidance resulting in the combined effect, joint infringement can be claimed.
V. Conclusion
Combination drug use has become a significant direction in current drug development. Providing patent protection commensurate with the contribution of combination drug inventions is a major challenge for China's patent practice. On the one hand, Chinese pharmaceutical companies are making great strides in innovative drugs, while on the other hand, protecting their innovations remains a pain point.
The legislative purpose of the patent law is to enhance innovation capabilities and promote scientific, technological, and economic development. Given the current legal practice's inability to fully support the pharmaceutical industry, appropriate legal adjustments are urgently needed to meet the demands of innovation and development. By appropriately adjusting relevant legal provisions to protect pharmaceutical innovations like combination drug use, it not only promotes the healthy development of the pharmaceutical industry but also aligns with the legislative intent of the patent law and government policies encouraging pharmaceutical innovation. It is hoped that legislative and judicial practices will quickly adjust relevant legal systems to better support the development of innovative drugs.
* Yuxuan Chang, Xiaolei Li, attorneys at law of Beijing Lungtin Law Firm
Footnotes
1. Pharmacology, Edited by Wang Naiping, Shanghai Science and Technology Press, 2006 edition
2. https://www.cn-healthcare.com/articlewm/20230422/content-1540380.html
3. Jing Yang · Heming Kang · Liyang Lyu · Wei Xiong. A target map of clinical combination therapies in oncology: an analysis of clinicaltrials.gov. Discover Oncology, Vol 14, (2023)
4. https://akesobio.com/cn/rd-and-science/pipelines/
5. https://www.inxmed.com/intro/25.html
6. https://www.hutch-med.com/sc/pipeline-and-products/our-pipeline/
7. https://www.inventisbio.com/pipeline/
8. Yalin Li, Swiss-Type Claim Research — A Study on the Patent Protection of Pharmaceutical Use Inventions, Master's Thesis of China University of Political Science and Law, October 2010
9. "Examination Guidelines 2010", Part II, Chapter 10, Sections 4.5.2 and 5.4
10. (2012)-IP-Administrative Litigation-No. 75 Retrial Decision
11. (2015) -IP-Administrative Litigation-No. 355 Decision
12. Invalidation Decision No. 561041
13. (2023)-Supreme Court-IP-Civil Action No. 2 Decision
14. (2022)京73民初314号民事判决书
15. The European Patent Convention (EPC), established in 1973 and effective as of 1978.
16. The European Patent Convention (2000), revised in 2000 and effective as of December 13, 2007
17. The European Patent Convention (2000), Article 83
18. Case law T1020/03
19. Article 54
(4) Paragraphs 2 and 3 shall not exclude the patentability of any substance or composition, comprised in the state of the art, for use in a method referred to in Article 53(c), provided that its use for any such method is not comprised in the state of the art.
(5) Paragraphs 2 and 3 shall also not exclude the patentability of any substance or composition referred to in paragraph 4 for any specific use in a method referred to in Article 53(c), provided that such use is not comprised in the state of the art.
20. Actavis v Eli Lilly [2017] UKSC 48
21. Sanofi-Aventis U.S. LLC v. Sandoz Inc (Civil Action 20-804-RGA)
22. Caraco Pharmaceutical Laboratories, Ltd. v. Novo Nordisk A/S, 566 U.S. 399 (2012)
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


