This year has been marked by robust efforts by the Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) to modernize food labeling in the United States. The agencies' regulatory agenda under the Trump Administration moves forward with many of the Obama Administration's food policy priorities (as we previously discussed here) and, at the same time, delays enforcement of other regulatory requirements to facilitate coordinated and less burdensome implementation of the regulatory changes by industry.
In January, FDA issued its Strategic Policy Roadmap, entitled "Healthy Innovations, Safer Families," which highlighted a goal to "empower consumers to make better and more informed decisions about their diets and health; and expand the opportunities to use nutrition to reduce morbidity and mortality from disease." 1 Consistent with FDA's Strategic Policy Roadmap, the first half of 2018 has included significant regulatoryishing Certain Reference Amounts Customaril actions, including:
- Implementing menu labeling rules;
- Extending the compliance deadline for implementing the updated Nutrition Facts labeling regulations and issuing several guidance documents to facilitate the modernization of nutrition labeling in the United States; and
- Unveiling the Nutrition Innovation Strategy and taking steps towards anticipated regulatory action in 2018.
In addition, on May 7, the USDA issued the long-awaited proposed rule on the National Bioengineered Food Disclosure Standard. This Advisory provides a brief recap of recent food labeling developments, a preview of what to expect in the coming months, and deadlines for open comment periods.
I. Recent Nutrition and Food Labeling Developments
As part of its 2018 Strategic Policy Roadmap, FDA announced its intent to implement a Nutrition Action Plan with the goal of "reduc[ing] preventable death and disease caused by poor nutrition by ensuring that consumers have access to accurate, useful information to make healthy food choices" and "foster development of healthier food options." FDA then proposed the following actions:
- Issue practical, substantive guidance to advance implementation of the menu labeling regulations;
- Provide guidance for industry to implement the new requirements for updating the Nutrition Facts label;
- Launch a new public education campaign to help consumers maximize the public health benefits of the Nutrition Facts label and new menu labeling provision; and
- Initiate a new, comprehensive action plan encompassing steps FDA will take to advance policies that better leverage nutrition and diet as ways to reduce morbidity and mortality from disease.
In the first five months of 2018, FDA has already tackled half of the plan.
A. Implementation of Menu Labeling Regulations Focusing on Education Instead of Enforcement
After several extensions to the deadline for compliance with FDA's menu labeling regulations, the rules became effective on May 7, 2018.2 The menu labeling regulations require restaurants and similar retail food establishments having 20 or more locations to provide calorie and other nutrition information to consumers at the point of purchase.
FDA has made clear that during the first year that the rules are in place the Agency will focus on education and not enforcement, seeking to work cooperatively with covered establishments to attain compliance with the rules. The Agency intends to "allow establishments a reasonable opportunity to make corrections for minor violations."3 FDA further intends to exercise enforcement discretion regarding the "calories from fat" declaration requirement because of its position that current science is supportive of the understanding that calories from fat are more relevant to the risk for chronic disease than overall caloric fat intake.4
FDA also finalized the Menu Labeling: Supplemental Guidance for Industry, which incorporates input from the public and industry stakeholders.5 The updated guidance addresses industry concerns about implementing the labeling requirements and advises on how to comply with the requirements. For example, the guidance provides photographic examples of ways to comply with the menu labeling requirements and graphical depictions of how to disclose calorie information for multiple items on one sign.
In addition to industry education, as part of the Agency's Nutrition Innovation Strategy, FDA will be rolling out a campaign focused on helping consumers better understand daily caloric requirements and how to make healthy food swaps so that they can benefit from the newly-available information. As FDA Commissioner Dr. Scott Gottlieb explained, the Agency's goal is to "implement these Congressional provisions in the most efficient, effective manner that both benefits consumers without placing unnecessary barriers on industry." 6
B. Compliance Deadline for the Nutrition Facts Label Pushed Back to January 2020
In response to stakeholder concerns about the time-intensive and burdensome nature of overhauling their labels to comply with the new food labeling requirements, FDA has once again delayed the compliance date for two final rules that affect the Nutrition Facts Label: (1) Food Labeling: Revision of the Nutrition and Supplement Facts Labels (the Nutrition Facts Label Final Rule) and (2) Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed At One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments (the Serving Size Final Rule). For an overview of the final rules, which were published on May 27, 2016, please read our firm's 7 Now, manufacturers with $10 million or more in annual food sales must comply starting on January 1, 2020, while manufacturers with less than $10 million in annual food sales must comply by January 1, 2021.
In deciding to extend this deadline, the Agency noted the importance of giving manufacturers time to use FDA guidance to address technical questions raised after issuance of the final rules, such as questions pertaining to fiber, on which FDA issued guidance in March 2018. In fact, since January, FDA has issued sixducts belonging in the product categ guidance documents in addition to menu labeling (discussed above) related to compliant labeling, including the following:
- Highly Concentrated Caffeine in Dietary Supplements (April 2018). FDA provides guidance to firms that "manufacture, market, or distribute dietary supplement products that contain pure or highly concentrated caffeine, or are considering doing so." FDA outlines its position that products that sell highly concentrated caffeine in bulk may be adulterated per section 402(f)(1)(A) of the Federal Food, Drug, and Cosmetic (FD&C) Act. The Agency has honed in on products that contain pure or highly concentrated caffeine in powder or liquid forms, since these products are often provided in bulk form. The products often contain multiple potentially lethal doses and require the customer to measure a safe serving.
- Scientific Evaluation of the Evidence on the Beneficial Physiological Effects of Isolated or Synthetic Non-Digestible Carbohydrates Submitted as a Citizen Petition (21 CFR 10.30) (February 2018). The guidance explains FDA's current thinking on information needed when submitting a citizen petition and the scientific approach the Agency plans to use for evaluating scientific evidence to determine whether an isolated or synthetic non-digestible carbohydrate that is added to food has a physiological effect that is beneficial to human health and, thus, can be legally declared on the nutrition facts label).
- Draft Guidance for Industry: Declaration of Added Sugars on Honey, Maple Syrup, and Certain Cranberry Products (February 2018). The guidance advises food manufacturers of the Agency's intent to exercise enforcement discretion regarding use of the symbol " " in the Nutrition Facts label to direct consumers to truthful and non-misleading statements on the package outside of the Nutrition Facts label. The symbol " " immediately follows the added sugars percent Daily Value information on single ingredient packages and/or containers of pure honey or pure maple syrup and on certain dried cranberry and cranberry juice products that are sweetened with added sugars and that contain total sugars at levels no greater than comparable products with endogenous (inherent) sugars, but no added sugars. Comments on the guidance are due by June 15, 2018.
- Proper Labeling of Honey and Honey Products (February 2018). The guidance advises, in a question and answer format, on how to properly label honey and honey products as required by sections 402 and 403 of the FD&C Act to avoid adulteration and misbranding. The intent of the guidance is to help ensure that consumers understand the nature of the product they are purchasing.
- Reference Amounts Customarily Consumed: List of Products for Each Product Category (February 2018) (providing examples of products belonging in the product categories included in the Reference Amounts Customarily Consumed (RACCs) per Eating Occasion tables (21 CFR § 101.12(b)).
- Serving Sizes of Foods That Can Reasonably Be Consumed At One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Small Entity Compliance Guide (February 2018). FDA provides additional guidance on how to comply with the updated Serving Size Final Rule.
FDA also plans to coordinate enforcement of the Nutrition Facts label regulations with USDA's implementation of the Bioengineering Disclosure Rule (discussed below).
C. USDA: Proposed National Bioengineered Food Disclosure Standard Rule
On May 7, USDA published its proposed rule for establishing a national bioengineered (BE) food disclosure standard (the NBFDS Rule).8 For more information on the 2016 law that mandated national BE food disclosures, please see our discussion on the Senate and House passage of the bill.9 Under the proposal, the compliance deadline would coincide with the compliance deadline for Nutrition Facts labeling (i.e., January 1, 2020, with the exception of small manufacturers, who must comply by January 1, 2021). Key components of the proposal include the definition of BE food, the types of food product covered by the rule, and the manner to disclose, which are highlighted below. USDA is accepting comments on the proposed rule until July 3, 2018.
Proposed Definition of a BE Food
Consistent with the 2016 BE food disclosure law, USDA's Agricultural Marketing Service (AMS) proposes to define a BE food as a "food that contains genetic material that has been modified through in vitro recombinant deoxyribonucleic acid (DNA) techniques and for which the modification could not otherwise be obtained through conventional breeding or found in nature."10 The proposed rule adds that foods that merely contain an incidental additive at an insignificant level, and that "does not have any technical or functional effect in the food" would not be considered a BE food.11
AMS has proposed and requested comment on three alternatives for foods that, although they contain a bioengineered substance, would be exempt from disclosure based on the following criteria:
- Food in which an ingredient contains a BE substance that is inadvertent or technically unavoidable, and accounts for no more than 0.9 percent by weight of that ingredient.12
- Food in which an ingredient contains a BE substance that is inadvertent or technically unavoidable, and accounts for no more than 5 percent by weight of that ingredient.13
- Food in which the ingredient(s) that contain a BE substance account for no more than 5 percent of the total weight of the food.14
AMS proposes to interpret the phrase, "inadvertent or technically unavoidable" as "insignificant amounts of a BE substance in a food that resulted from the coexistence of BE and non-BE foods in the supply chain."15
Proposed Exemptions from the NBFDS Rule
In general, the NBFDS Rule applies to foods subject to the labeling requirements of the FD&C Act, and certain foods subject to labeling requirements of the statutes administered by the USDA's Food Safety and Inspection Service (FSIS): the Federal Meat Inspection Act, the Poultry Products Inspection Act, and the Egg Products Inspection Act. The rule, however, would exclude the following foods from the disclosure standard:
- Articles intended for animal consumption, such as pet food;
- Foods that are certified organic under the National Organic program;
- Foods derived from animals who consumed feed produced from, containing, or consisting of a BE substance, from the disclosure standard;
- Food served in restaurants or similar retail food establishments;
- Very small food manufacturers (annual receipts of less than $2,500,000) from the disclosure requirements;16 and
- Certain food subject to the FSIS statutes, such as pork or eggs.
In particular, food subject to the FSIS statutes, such as pork or eggs, would only fall under the NBFDS Rule if one of the following two conditions is met:
- The most predominant ingredient of the food would independently be subject to the FD&C Act's labeling requirements, or
- The most predominant ingredient of the food is broth, stock, water, or a similar solution, and the second most predominant ingredient of the food would independently be subject to the FD&C Act's labeling requirements.17
For example, if meat, poultry, or egg is the first ingredient in a food product with multiple ingredients, the product would not be subject to the NBFDS Rule. By contrast, if meat, poultry, or egg was the third (or lesser) most prominent ingredient, the product would be subject to the NBFDS Rule.
To assist regulated entities in determining whether they need to disclose the BE status of a food or food product, AMS developed two lists of commercially available BE foods that are subject to disclosure, according to whether the foods are:
- "Highly adopted" commercially available BE foods, which are those that have an adoption rate of 85 percent or more in the US market. Examples include: canola, corn (field), cotton, soybean, and sugar beet.
- "Not highly adopted" commercially available BE foods, which are those that have an adoption rate of less than 85 percent in the US market. Examples include: apple (non-browning cultivars), corn (sweet), papaya, potato, and squash (summer varieties).
If a food is on one of the two lists, the regulated entity would need to disclose the BE status of the food. In the case of a food or food product that contains an ingredient on one of the two lists, but is not accompanied by a BE disclosure, regulated entities must maintain documented verification that the product is not, or does not contain a BE food.18
Proposed Manner to Disclose the BE Status of a Food
The proposed rule offers several options for regulated entities to disclose the BE status of a food–(1) text disclosure, (2) symbol disclosure, or (3) electronic or digital disclosure. The manufacturer or importer must ensure that the proper disclosure is provided on the food label if the product is packaged before receipt by the retailer.19 For foods sold or packaged in bulk by a retailer, the retailer must ensure that the proper disclosure accompanies the BE foods.20
- Text Disclosure.21 The list on which a BE food is included–either "highly" or "not highly adopted"–governs the disclosure statement that may be conveyed about the food. The proper textual disclosure for a "highly adopted" BE food or BE food ingredient is, "bioengineered food" or "contains a bioengineered food ingredient". The phrase, "bioengineered food", applies to raw agricultural products (e.g., corn) and processed products that only contain BE food ingredients (e.g., cornmeal). "Contains a bioengineered food ingredient" applies to all other foods. For a "not highly adopted" BE food, entities may use the following statements: "bioengineered food", "may be bioengineered food", "contains a bioengineered food ingredient", or "may contain a bioengineered food ingredient." AMS has requested comments on the proposed text disclosures, including with regard to the use of "may be" or "may contain" disclosures.
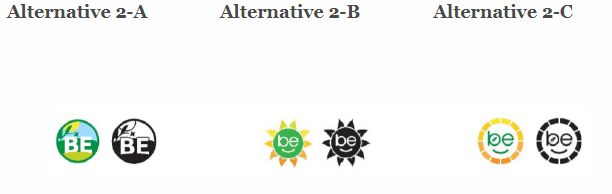
- Symbol
Disclosure.22 AMS has proposed, and requests
comments on, three alternatives for the symbols that may be used to
indicate the BE status of a food.
- Electronic or Digital Link Disclosure.23 Regulated entities may also disclose the BE status of a food through electronic mechanisms such as scanning a code with a smartphone or other device. In this case, regulated entities must also include the statement, "scan here for more information" or similar language reflecting the technology used to access the information, and provide at telephone number with instructions to "call for more information."
In addition to the disclosure methods described above, small food manufacturers (annual receipts greater than $2,500,000 but less than $10,000,000) have two additional disclosure options: (1) providing a telephone number accompanied by the phrase, "call for more information", or (2) an internet website address, accompanied by the phrase, "visit [website] for more food information."
Disclosures should be placed on the package in accordance with proposed 21 CFR § 66.100(d). Note, that for bulk foods, such as seafood or fresh produce, the disclosure must be provided on signage or other materials that "allows consumers to easily identify and understand the bioengineered status of the food."24
II. What's Next?
On March 29, 2018 FDA Commissioner Gottlieb announced the FDA Nutrition Innovation Strategy, intended to "take a fresh look at what can be done to reduce preventable death and disease related to poor nutrition." While the strategy is still in the early stages of development, key elements of the strategy are supposed to include "modernizing claims, modernizing ingredient labels, modernizing standards of identity, implementing nutrition facts label and menu labeling, and reducing sodium." Since March, FDA has taken steps towards agency action in these areas, which we have discussed below and will continue to monitor.
A. "Healthy" and "Natural" Food Labeling
Updating the standards governing the use of "healthy" and "natural" in food labeling remains a priority for FDA. "Healthy" was first defined by the Agency in 1993, but much has changed in dietary and clinical guidelines in the intervening years. FDA's current approach to the use of the term "healthy" in food labeling is set forth in a 2016 Agency guidance.25 The guidance conveyed the Agency's intention to exercise enforcement discretion for foods that bear "healthy" labeling and exceed the limits for total fat, provided that monounsaturated and polyunsaturated fats are present in larger amounts than saturated fats.
FDA also intends to exercise enforcement discretion for foods rich in potassium or vitamin D, despite their absence from the current regulations. Last year, FDA held a public meeting to seek stakeholder input on its plan to update the regulatory standards for using "healthy" as an implied nutrient content claim in human food labeling, as we previously discussed here. FDA noted its intent to revise requirements for "healthy" claims this year in its Strategic Plan for 2018. Dr. Gottlieb reiterated the Agency's focus on use of the term "healthy", explaining that the Agency will be taking into consideration a broad range of issues, such as availability of information about nutrients, as the Agency pursues rulemaking to update the definition of "healthy", "so it's based on nutrition criteria and food considerations that are more up-to-date than those being used for the current definition."26 He also discussed that FDA will be considering how to depict "healthy" on food packaging for consumers to easily locate this information. FDA will be soliciting stakeholder input on whether a standard icon or symbol for "healthy" should be developed for use on food packages.27
With regard to use of the term "natural" in food labeling, as we previously discussed here, the Agency's current approach is that a food labeled as "natural" should have "nothing artificial or synthetic (including all color additives regardless of source) included in, or added to the food."28 Dr. Gottlieb has also reiterated the Agency's commitment to establishing a definition for "natural." For example, at the National Food Policy Conference on March 29, 2018, Dr. Gottlieb discussed his viewpoints and the Agency's progress, recognizing the lack of clarity surrounding the term, "natural", and explaining that like other claims about FDA-regulated products, such claims must be "true and based in science."29 Action on these claims is expected this year.
B. New Qualified Health Claims
Modernizing health claims, including how the Agency reviews health claims that it receives from industry, is a key component of the Nutrition Innovation Strategy. The Agency intends to streamline the review process for qualified health claims, in part due to the time-consuming nature of claim review. As Dr. Gottlieb explained, "We have a number of [qualified health claims] in the queue right now, and they take a significant time to review."30 Accordingly, FDA plans to "triage requests based on their public health significance and prioritize those that are the most meaningful and science-based."31 FDA points to its recently-permitted qualified health claim linking early peanut introduction and reduced risk of developing a peanut allergy as an example of its new approach to health claims.32 The Agency hopes that improving its review process and prioritizing requests would "prioritize those qualified health claims that are most likely to have a health benefit, and that are based on the strongest science would encourage industry to submit their strongest, most significant, claims for review and to discourage submission of claims with little value."33
C. Modernizing Standards of Identity
FDA signaled that modernizing Standards of Identity (SOIs) would be an important Agency priority over the coming year by including this issue in the Nutrition Innovation Strategy. SOIs are "mandatory requirements related to the content and production of certain food products," including yogurt, bread, jams and more.34 FDA intends to issue a Request for Information to help guide the Agency in developing a strategy as it evaluates existing SOIs. Through these efforts, the Agency seeks to "maintain the basic nature and nutritional integrity of products while allowing industry flexibility for innovation to produce more healthful foods."35
According to Commissioner Gottlieb, key products impacted by FDA's evaluation of SOIs are expected to include dairy and yogurt products, based on requests made by the International Dairy Foods Association.36 In response to a broad request for comments to assist FDA in its regulatory reform activities, the International Dairy Foods Association submitted comments to FDA's Center for Food Safety and Applied Nutrition (CFSAN) setting forth 24 priority issues for CFSAN Regulatory Reform, including a number of requests for modernizing SOIs for milk, yogurt, and cheese.37 In particular, IDFA specifically requested that FDA finalize a 2009 proposed rule to modernize the yogurt SOIs.38 We will monitor Agency developments to ensure clients are aware of opportunities to comment on proposed agency action.
D. FDA Focus on Voluntary Sodium Reduction
Advancing guidance on dietary sodium reduction targets is another key component of the Agency's Nutrition Innovation Strategy. As we previously discussed here, on June 2, 2016, FDA released draft guidance for industry on the Agency's effort to work with food companies and restaurants to gradually reduce sodium levels in food. Once finalized, the draft guidance document will provide short-term (two years) and long-term (ten years) voluntary mean and upper-bound targets for sodium concentrations in categories of commercially processed, packaged, and prepared foods.
The proposed short-term targets aim to reduce sodium intake from the current average intake of over 3,400 mg/day to 3,000 mg/day, while the long-term goal is to reduce sodium intake to 2,300 mg/day. FDA is currently working on addressing over 150 public comments on the targets, and intends to align its efforts with the Dietary Reference Intake activities being conducted by the National Academies. According to Dr. Gottlieb, FDA plans to release the updated short-term targets in 2019 and will continue engaging on the longer-term targets.39
***
In the first half of 2018, FDA and the USDA have taken significant steps to fulfill the Obama and Trump Administrations' commitment to modernizing the country's food labeling system. Companies engaged in the development, marketing, and sale of food intended for use in the United States should anticipate forthcoming opportunities to comment on proposed agency action intended to (1) educate consumers about the relationship between diet and chronic disease and (2) encourage food (and recipe) choices by consumers (and industry) that promote a healthy lifestyle. Our team will continue to monitor these developments and advise you on pathways to both comment on agency proposals and comply with finalized regulatory guidance and regulations. In the interim, please feel free to contact us with any questions about the topics discussed in this advisory.
© Arnold & Porter Kaye Scholer LLP 2018 All Rights Reserved. This Advisory is intended to be a general summary of the law and does not constitute legal advice. You should consult with counsel to determine applicable legal requirements in a specific fact situation.
Footnotes
1 Healthy Innovation, Safer Families: FDA's 2018 Strategic Policy Roadmap, Jan. 2018, at 15.
2 FDA Extends Menu Labeling Compliance Date to 2018, May 1, 2017. See 21 CFR § 101.11.
3 FDA, PowerPoint Presentation on Menu Labeling.
4 FDA Guidance for Industry, Menu Labeling: Supplemental Guidance for Industry, May 2018, at 4.
5 Id.
6 Statement from FDA Commissioner Scott Gottlieb, M.D., on the public health benefits from enactment of menu labeling, May 7, 2018.
7 Id.
8 National Bioengineered Food Disclosure Standard, 83 Fed. Reg. 19860, (May 4, 2018).
9 National Bioengineered Food Disclosure Standard, Pub. L. 114-216 (July 29, 2016).
10 Proposed 21 CFR § 66.1, definition of a bioengineered food.
11 Id.
12 Proposed 21 CFR § 66.5(c), Alternative 1-A.
13 Id., Alternative 1-B.
14 Id., Alternative 1-C.
15 83 Fed. Reg. 19860, 19869.
16 Proposed 21 CFR § 66.5(a)-(b), (d)-(e).
17 Proposed 21 CFR § 66.3(b)(2). Note that predominance refers to the position of an ingredient on a list of ingredients, and is determined by the weight of the ingredient in the product, per 21 CFR § 101.4(a)(1).
18 83 Fed. Reg. 19860, 19871.
19 Proposed 21 CFR § 66.100(a).
20 Id.
21 See proposed 21 CFR § 66.102.
22 See proposed 21 CFR § 66.104.
23 See Proposed 21 CFR § 66.106.
24 Proposed 21 CFR § 66.114.
25 See Guidance for Industry: Use of the Term "Healthy" in the Labeling of Human Food Products, Sept. 2016.
26 Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018.
27 Id.
28 "Natural" on Food Labeling, FDA.
29 Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018 ;Id.
30 Id.
31 FDA's Nutrition Innovation Strategy, available at https://www.fda.gov/Food/LabelingNutrition/ucm602651.htm (updated Mar. 29, 2018); Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018, available at https://www.fda.gov/NewsEvents/Speeches/ucm603057.htm.
32 FDA Acknowledges Qualified Health Claim Linking Early Peanut Introduction and Reduced Risk of Developing Peanut Allergy, Sept. 7, 2017, available at https://www.fda.gov/Food/NewsEvents/ConstituentUpdates/ucm575001.htm.
33 Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018, available at https://www.fda.gov/NewsEvents/Speeches/ucm603057.htm.
34 FDA's Nutrition Innovation Strategy, available at https://www.fda.gov/Food/LabelingNutrition/ucm602651.htm (updated Mar. 29, 2018).
35 Id.
36 Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018.
37 IDFA's 24 Priority Issues for FDA CFSAN Regulatory Reform, Feb. 2, 2018.
38 Id.
39 Reducing the Burden of Chronic Disease, Remarks by Scott Gottlieb, M.D., Commissioner of Food and Drugs, National Food Policy Conference, Washington DC, Mar. 29, 2018.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




