In Allergan USA, Inc v Sun Pharmaceutical Indus. Ltd, Appeal No. 2024-1061, August 13, 2024, the Federal Circuit reversed the district court's holding that Allergan patent, U.S.P. 7,741,356 ('356) was invalid for obviousness-type double patenting over USPs 11,007,179, 11,090,291, and 11,311,516. The relationship between the patents is shown below:
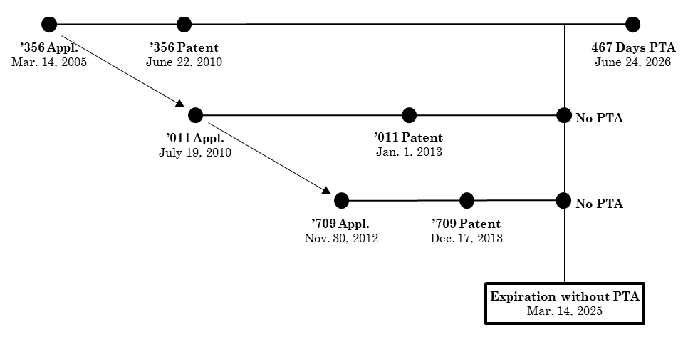
The '356 patent was entitled to significant PTA but because of the PTE it received, Allergan effectively disclaimed all but 487 days of the PTA. The total extension PTE plus PTA resulted in an expiration date of May 27, 2029, 15 years from the '356 issue date -- the maximum extension possible.
Sun argued that '356 claim 40 was invalid for ODP over claim 33 of the '011 patent and claim 5 of the '709 patent. In the district court Allergan argued unsuccessfully that as the first patent filed and issued claiming eluxadoline it was not subject to ODP. The district court rejected the argument because in its opinion one compares expiration dates and not either issuance dates or filing dates, citing Gilead Scis., Inc. v. Natco Pharma Ltd., 753 F.3d 1208, 1215–17 (Fed. Cir. 2014), and In re Cellect, LLC, 81 F.4th 1216, 1228–29 (Fed. Cir. 2023)). The Federal Circuit disagreed holding that the first filed and first issued patent was not subject to a double patenting invalidity. The concept is the first issued patent is the reference for an ODP rejection. This makes sense because for a double patenting rejection to be proper, there must first be a patent that has an earlier issue date than the patents subject to the ODP, after all it is double patenting which requires at least two patents. As the first to issue, the '356 patent was the proper reference for the ODP rejection.
Sun relied upon In re Cellect,81 F.4th 1216, 1226 (Fed. Cir. 2023)) that patent expiration dates controlled. However, Sun and the district court ignored Cellect's statement:
ODP "is intended to prevent a patentee from obtaining a timewise extension of patent for the same invention or an obvious modification thereof" and prevents an inventor from claiming a second patent for claims that are not patentably distinct from the claims of a first patent. [Emphasis added]
In Cellect the first filed patent which was the reference patent did not receive PTA and it expired before the later filed child patents which received PTA. The existence of the PTA did not create the double patenting issue since it would have existed even if no PTA had been granted in the later filed applications, since without a terminal disclaimer it would have been possible for the patents to have different owners. One of the requirements of a terminal disclaimer is that common ownership be maintained between the patents.
It is important in filing continuation applications where the claims are obvious over the parent application that a terminal disclaimer is necessary. Do not rely on the patent examiner's decision not to issue an ODP rejection. The Examiner's decision not to enter an ODP rejection is no guarantee that one cannot be successfully raised in a subsequent action. Also, remember it's the subsequent patents which are invalid and not the first.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


