Introduction
In a suit for infringement of Roche's Patent No. IN 334397 titled 'COMPOUNDS FOR TREATING SPINAL MUSCULAR ATROPHY', a species patent inter alia claiming a compound having an International Non-Proprietary Name ('INN') Risdiplam1, against Natco for preparing for commercial production of Risdiplam API; the Single Judge of the Delhi High Court by order dated 24 March 20252 denied grant of interim injunction in favour of Roche mainly on the basis that Natco was able to demonstrate prima facie that there exists a credible challenge to the validity of Patent No. IN 334397.
However, shortly thereafter the Hon'ble Division Bench of the Court, on an appeal3 by Roche against the Single Judge's order, directed on 26 March 2025, that the status quo regarding launching of Natco's product be maintained. The said status quo order continues to operate till the date of writing this article. The Division Bench is considering the appeal, and the next date of hearing is 21 April 2025.
Background of the case
The Suit Patent in dispute, i.e., IN 334397 ('IN'397') was granted in favor of Roche on 11 March 2020. IN'397 is a species/selection patent for the product 'Risdiplam' which is essentially a compound used in the treatment of a rare disease i.e., Spinal Muscular Atrophy ('SMA'). US 9969754 ('US'754') is the US counterpart of IN'397. Further, US 9586955 ('US'955') is the corresponding patent for PCT patent application no. PCT/US2013/025292, published as WO 2013/119916 A2 ('WO'916'). WO'916 is the international genus patent for the Suit Patent. Roche also filed an application for Patent Term Extension ('PTE') in respect of US'955 before the United States Patent & Trademark Office ('USPTO').
Dispute arose when Roche became aware of a listing for Risdiplam on Natco's website. Upon investigating further, Roche found that Natco was preparing for the commercial production of Risdiplam. Additionally, Natco was also pursuing a patent application i.e., 202241055182, for manufacturing Risdiplam. Aggrieved by the same, Roche instituted the present suit alleging infringement of their Suit Patent by Natco. Owing to the public interest angle qua accessibility of 'Risdiplam' coupled with its high costs, two intervenors though not formally impleaded but were heard by the Ld. Single Judge.
Roche's arguments
Roche asserted that the Suit Patent inter alia claiming Risdiplam, which is set to expire in May 2035, is valid and enforceable. Natco violated their patent rights because they already initiated commercial production and are in the process of launching Risdiplam, thus admitting infringement.
Roche asserted that Risdiplam cannot be anticipated or rendered obvious based on the disclosure in WO'916 as it does not specifically teach or disclose Risdiplam and no one can find Risdiplam as a specific example in WO'916. It was asserted that a person of ordinary skill in the art ('POSA'), not being aware of Risdiplam without hindsight bias, could not narrow down to the structure of Risdiplam from various Markush Structures in WO'916.
Further, it was argued that the Suit Patent enjoys a strong presumption of validity being an old patent filed in the year 2016 and claiming priority since the year 2014. Corresponding patents with claims directed to the specific compound i.e., Risdiplam were granted in about 60 countries, even after the consideration of WO'916 in such jurisdictions, and the same have not been revoked/invalidated in any jurisdiction. Moreover, no challenge to the novelty/anticipation of the Suit Patent was raised by the Indian Patent Office.
Regarding public interest and accessibility of Risdiplam, Roche stressed that the expenses borne during the development of a new drug are exorbitant unlike Natco, which being a generic only had to bear costs for manufacturing. Therefore, striking a balance is necessary between the interest of innovators and the generic industry.
Natco's arguments
Natco argued that both, the genus- WO'916, and Indian Species Patent, IN'397, relate to compounds for the treatment of the same condition, namely SMA. Further, Compound 809 disclosed in the prior art document WO'916 was referred, to demonstrate that Risdiplam was already disclosed.
Regarding the differences between the chemical structures of compound 809 and Risdiplam, Natco asserted that any modifications or substitutions which result in the same chemical and physical properties, and are necessary to arrive at the compounds claimed in the Suit Patent from the compounds disclosed in the WO'916, are routine and predictable by a person skilled in the art, being disclosed in prior art itself.
Natco while highlighting the issue of 'evergreening of patents' submitted that, a species/selection patent can be granted despite the grant of a genus patent, only if it is demonstrated that the species patent has significant technical advancement and enhanced therapeutic efficacy over the genus patent. However, the Suit Patent fails to disclose any such advantage over WO'916.
Natco argued that Roche had received a Patent Term Extension ('PTE') for US'955 premised on an express admission that Risdiplam is a new medication with its discovery attributable to US'955. On the aspect of public interest and accessibility of Risdiplam it was argued that while Natco will be manufacturing the drug in India, Roche will be only importing Risdiplam into the country thus, its intent being to only profit off the drug.
Intervenors' arguments:
The intervenors (both patients of SMA) primarily argued about the unaffordability of Risdiplam, that made the drug inaccessible to several patients suffering from SMA. The intervenors highlighted the drug's exorbitant price which resulted from the patent monopoly exercised by the Roche thus, keeping Risdiplam beyond the reach of many.
Single Judge's findings
In an order spanning several pages, the Ld. Single Judge ruled against granting an interim injunction in favour of Roche, largely based on the following considerations:
I. On the aspect of prima facie case:
1. Anticipation by Prior Publication- Section 64(1)(e) of the Patents Act, 1970 (Act):
The primary issue analysed by Single Judge was whether Risdiplam is explicitly or implicitly disclosed in WO'916, which has been cited as prior publication/prior art by Natco. While citing Bayer Healthcare LLC v. NATCO Pharma Limited4, and Schering Corporation v. Geneva Pharmaceuticals, Inc5, the Single Judge held that disclosure can be implicit/inherent, and there is no stringent rule that it ought to be explicit in nature. Thus, if from the prior art, it can be inferred that there is disclosure, though implicit/inherent, that would be a valid ground for challenging the validity of a patent based on lack of novelty.
The Ld. Single Judge also referred to a lawsuit filed by Roche against Natco in the United States, for infringing the US'955, by launching Risdiplam. Thus, by asserting patent rights over Risdiplam under US'955, as per Roche's own admission, Risdiplam is covered by US Patent US'955. The Judge also placed reliance on the US FDA Orange Book Listing for Risdiplam, wherein the product Risdiplam is categorically claimed for US Genus Patent, US'955, which is equivalent to the International Genus Patent, WO'916.
The Ld. Single Judge reasoned that for the purposes of establishing a prima facie view and for considering the issue regarding credible challenge being raised by the defendant while considering the application for grant of interim injunction, the various statements made by the plaintiffs in foreign jurisdiction are material and relevant.
The Ld. Single Judge also referred to the Hon'ble Division Bench's (DB) findings in Astrazeneca AB & Another vs. Intas Pharmaceutical Limited6, and held that in cases where all or some of the inventors are common, the test while considering 'anticipation by publication', would be from point of view of 'person in the know', and not in the context of 'person ordinarily skilled in the art'. The Ld. Single Judge held that all the four inventors of the Suit Patent, are also the inventors of the International Genus Patent, besides the other inventors of the International Genus Patent and therefore, pertinent for the purposes of considering an application, wherein an interim relief is sought.
Accordingly, the Single Judge held that Natco raised a prima facie credible challenge to the validity of the Suit Patent regarding the issue of anticipation by prior publication.
2. Lack of inventive step – Section 64(1)(f) of the Act
The Ld. Single Judge referred to the Hon'ble Division Bench's (DB) findings in Astrazeneca AB & Another vs. Intas Pharmaceutical Limited7, wherein the DB held 'when the inventor is the same, the tests of 'obvious to person skilled in the art', cannot be in the context of person ordinarily skilled in the art', but has to be seen in the context of a person in the know'. Observing that at least four inventors of WO'916 were common inventors in the suit patent as well, the Ld. Single Judge remarked that the issue of obviousness would have to be assessed from the point of 'a person in the know'.
Thereafter, the Ld. Single Judge drew an analogy between the chemical structures of Risdiplam and Compound 809 from WO'916. The Ld. Single Judge observed that the major difference in the structures is the presence of Nitrogen (N) in Risdiplam and Carbon-Hydrogen (C-H) group in Compound 809. It was observed that WO'916 discloses 835 compounds of which, Pyrimidine is a constituent in almost all the compounds, including, Compound 809. Pyridine is also a constituent in most of the compounds. As per the scientific definition, Pyridine has just one Nitrogen atom, whereas, Pyrimidine, has two Nitrogen atoms. On account of myriad occurrences of the Nitrogen atom in the various compounds, the Ld. Single Judge held that it is prima facie established that it would have been obvious to a person skilled in the Art/person in the know that Nitrogen is a dominant component of most of the compounds disclosed in WO'916 and such person skilled in the Art/ person in the know would have easily been motivated to use the Nitrogen atom instead of the Carbon atom, while looking at Compound 809 in the International Genus Patent. On this basis, it was held that Natco has prima facie established that the compounds claimed in the Suit Patent represent routine optimization of compounds disclosed in the prior art. Further, it was noted that it is common practice in the field of pharmaceuticals to make iterative modifications to chemical structures in order to improve properties such as potency, selectivity or metabolic stability.
Supplementing the foregoing findings, the Ld. Single Judge also analysed the concept of 'Bioisosters' while also referring to 'Grimms Hydride Displacement Law', a hypothesis devised in 1925 for explaining Bioisosteres. As per the hypothesis Nitrogen and C-H are in the same group of isosteres and often considered as bioisosteres, thus the Ld. Single Judge held that substitution of C-H group with Nitrogen would be obvious to a 'person in the know' or a person skilled in the art working in the domain of medicinal chemistry for optimization of compounds disclosed in the prior art i.e., WO'916.
Further, at the interim stage, the Ld. Single Judge did not consider the comparative data demonstrating the 'Effective Concentration' value (EC1.5x) of compounds described in the complete specification of the Suit Patent, as the data was heavily contested by Natco. The Court opined that the data would have to be examined further based on expert testimony and the same must done at the trial stage.
Thus, the Ld. Single Judge was of the prima facie view that Natco had been able to establish vulnerability of the suit patent on account of lack of inventive step based on the disclosure in the cited prior art document.
Bioisosterism – A deeper analysis
Bioisosters' are defined as subunits or groups or molecules which possess physicochemical properties of similar biological effects8. The concept of Bioisosterism refers to replacing atoms or groups with others that have similar physical or chemical properties to achieve similar biological effects. This phenomenon is increasingly being used in drug design for developing new compounds that are therapeutically active9. In Chemistry, Grimms Law describes the similarity between groups which have the same number of valence electrons but different number of atoms10. The law also points out similarities in groupings of element in the same row of the periodic table.
The Ld. Single Judge held for a 'person in the know', in the field of medicinal chemistry it would be obvious to substitute the C-H group with Nitrogen, to further explore the effects of such substitution on the drug's biological activity by perusing the prior art document (genus -WO'916). Chemical structures of Compound 809 and Risdiplam are reproduced hereinbelow for reference.
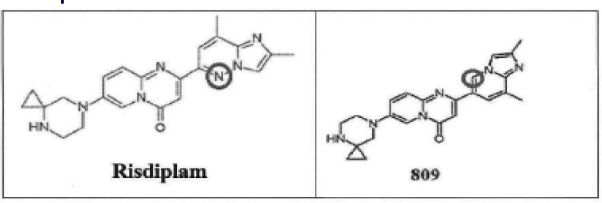
In the present case, the distinguishing feature of Risdiplam is in providing a solution to the problem of treating SMA. However, while making such an observation on the aspect of obviousness of the Suit Patent, it appears the Ld. Single Judge may not have considered whether this information was already available with a 'person in the know' about the probable impact of such a substitution (C-H to N) on the pharmacological activity of the drug in question.
In T 0467/94, the Board of Appeals, European Patent Office ('Board') while dismissing an objection on lack of inventive step based on bioisosteres, held that- 'In this context, the Examining Division held in their decision that the replacement of the alkoxy group by the methoxypropoxy group would have been obvious in the light of the concept of modern bioisosterism considering that an -O- atom and a -CH2- group were classical isosters. However, in the Board's judgment, when deciding upon inventive step in relation to pharmacologically active compounds, it is not essential whether a particular substructure of a compound could be replaced by another known isosteric one, but whether information was available on the impact of such a replacement on the pharmacological activity of the specific group of compounds concerned............... In the present case, the Examining Division did not provide any evidence that the replacement a -CH2- subgroup in said alkoxy substituent of the group of compounds defined in documents (2) and (4) by an -O- atom would have no substantial influence on their pharmacological properties. Moreover, documents (2) and (4) - as indicated above - unambiguously disclose that at the 4-position of the pyridinium ring only particular substituents are suitable, so that the skilled person would have expected that this is an essential requirement for having the desired anti-ulcer properties.'
In T 0643/96, the Board held that –
'4.2.3.3. The above quoted argument of the Examining Division (see No. 4.2.2) amounts in fact to an allegation that the existing structural differences between the compounds known from document (1) and those now claimed are so small that a skilled person would have known that such differences have no essential bearing on the properties important for solving the technical problem (T 0852/91, No. 8.2 of the Reasons for the Decision, not published in the OJ EPO). The validity of this argument hinges on the applicability of the concept of bioisosterism from which the skilled person would have drawn this knowledge.
The Board agrees that this concept belongs to the common general knowledge of those skilled in the art but, in the Board's judgement, it has to be applied with caution when deciding upon inventive step. In the field of drug design any structural modification of a pharmacologically active compound is, in the absence of an established correlation between structural features and activity, a priori expected to disturb the pharmacological activity profile of the initial structure. This holds true also for an alleged case of bioisosterism, which is one option of a structure-activity relationship, as long as it is not an established case of bioisosterism (see also T 0548/91, No. 6.4 of the Reasons for the Decision, not published in the OJ EPO).
A careful evaluation of all relevant circumstances is therefore required as to whether or not that a priori assumption can indeed be overcome with the aid of the concept of bioisosterism (which in essence is not a law of nature of general validity but rather an empirical rule, which in each particular case needs to be experimentally verified in order to establish whether or not it fits).
In document (3) the concept of bioisosterism is discussed. It is explained that in any bioisosteric replacement a considerable number of different, independent parameters could be considered: 'The extent to which the replacement is useful will depend upon which of these parameters is important and which ones the bioisostere can best mimic ... Usually one will not know which role(s) the various parts of the molecule play(s) in its action and this determination will be part of the structure-activity study'. The document concludes, inter alia, that 'Whether the same or a different biological activity results from the replacement will be governed by the role(s) which that moiety fulfils in the molecule and whether parameters affecting that role have been disturbed' (page 565, lines 16 to 19, and the last three lines; page 566, lines 3 to 5).
This clearly confirms that the concept of bioisosterism - at least in the circumstances of this case - is to be considered at most as providing some general guidance to the skilled person developing a research program in the particular pharmacological field, but certainly not as a pointer to the solution to the existing technical problem as now claimed (see also T 0309/91, No. 4.2.4 of the Reasons for the Decision, not published in the OJ EPO).
4.2.3.5. However, when deciding on inventive step in relation to pharmacologically active compounds, what is essential is not whether a particular sub-structure of a chemical compound is replaced by another known isosteric one, but whether information was available on the impact of such a replacement on the pharmacological activity profile of the (group of) specific compound(s) concerned. The Examining Division did not refer to, nor is the Board aware of, such information.
In T 0156/95, the Board held that –
'The Board accepts that =N= and =C- are well known isosteric groups. However, when deciding on inventive step in relation to pharmacologically active compounds, what is essential is not whether a particular sub-structure of a chemical compound is replaced by another known isosteric one, but whether information was available on the impact of such a replacement on the pharmacological activity profile of the (group of) specific compound(s) concerned.'
Therefore, it appears from the order that the Ld. Single Judge has not considered whether a person skilled in the art had any information based on any disclosure, as to the impact on the pharmacological activity profile of the compounds, on replacing the C-H group by the Nitrogen. Accordingly, it appears that the Court did not consider the nuance of applying the bioisosterism concept as has been applied in the EU.
On the ground of Section 64(1)(d) (non-patentability), the Ld. Single Judge observed that no finding could be given at this stage, as it was not argued by Natco. On the grounds of Section 64(1)(j) (misrepresentation) and Section 64(1)(m) (Section 8 non-compliance), the Ld. Single Judge did not give any finding on the basis that it is subject matter of trial.
II. On the aspect of balance of convenience
In assessing balance of convenience at the interim stage, the Ld. Single Judge noted that Roche does not manufacture their drugs in India but imports the same. In contrast, Natco intends to manufacture the drug in India and make the product available at a price that is nearly 80-90% lesser than Roche's price. Further, the Court noted that Roche's Patient Assistance Program ('PAP') covers a very miniscule number of patients, compared to the number of patients who are suffering from SMA in India. Therefore, it was held that Roche's PAP does not resolve the issue of accessibility of the drug in question to SMA patients and therefore, it cannot be stated that balance of convenience for grant of injunction, lies in Roche's favour.
III. On the aspect of irreparable damage/prejudice
In evaluating irreparable damage at the interim stage, the Ld. Single Judge observed that Roche is importing the drug at highly exorbitant price, which shows that Roche intends to monetize the drug, and therefore, Roche can be compensated in damages if they succeed post-trial. Accordingly, the Ld. Single Judge held that if the injunction is refused, no prejudice will be caused to Roche.
IV. Public interest
The Ld. Single Judge after considering the aspects of unaffordability and inaccessibility of Risdiplam, held that that the impact on public interest in the grant of an interim injunction would have to be considered, particularly in the case of pharmaceutical inventions. The Ld. Single Judge noted that a drug which is the only one available for treatment in India, for a rare disease, its availability to the public at large at very economical and competitive prices, is a material factor which a Court should consider at the time of dealing with an application for interim injunction.
Conclusion
The order delivered by the Ld. Single Judge, particularly the analysis of inventive step, may be wanting, especially in applying the concept of bioisosterism.
Despite evidence of enhanced efficacy, improved pharmacokinetic profile, and therapeutic advantages over known compounds, the Court appears to have adopted a simplistic assessment of inventive step based on a simpliciter application of the bioisosterism concept. The order leans heavily on the 'obvious to try' standard without acknowledging the specific challenges and unpredictability inherent in pharmaceutical research. The order appears to indicate that once a genus or class of compounds is known, any advancement within that class is routine optimization — an undesirable precedent that fails to consider the unpredictable and uncertain nature of drug development process.
The order, while detailed and ostensibly rooted in public interest, raises concerns about the way the credible challenge test is applied, especially in aspects of substantive technical assessment concerning inventive step.
Footnotes
1. Risdiplam is the API in the Roche's commercial product, which is marketed in various countries worldwide, including, India, under the brand name, 'EVRYSDI®'. Risdiplam is an oral prescription medicine indicated for the treatment of SMA in patients two months of age or older. SMA is a rare genetic neuromuscular disorder caused by the mutation of the Survival Motor Neuron 1 gene, leading to a deficiency of SMN protein, which affects motor nerve cells, diminishing the ability to walk, sit, eat and breathe.
2. 2025:DHC:1907
3. FAO(OS) (COMM) 43/2025
4. 2023 SCC OnLine Del 3921
5. 339 F.3d 1373 (2003)
6. 2021 SCC OnLine Del 3746
7. 2021 SCC OnLine Del 3746
8. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design, Lídia Moreira Lima and Eliezer J. Barreiro, Current Medicinal Chemistry, 2005, 12, 23-49
9. Supra 8
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



