On September 13, 2023, a manufacturer of radiology software solutions – Annalise.ai – announced that FDA granted a breakthrough designation for the first time for a radiology triage software medical device. The company notes in the press release that the breakthrough device designation is for an obstructive hydrocephalus software tool.
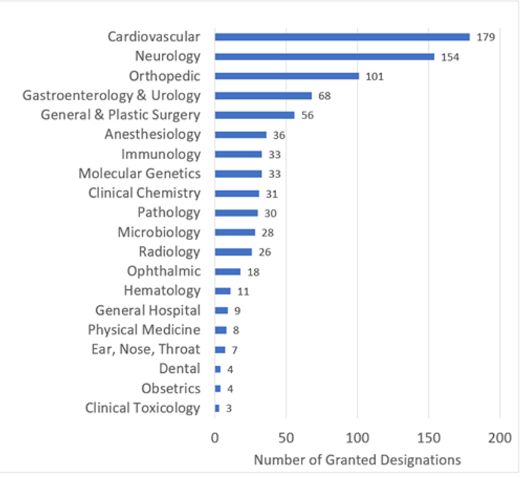
Although the company did not provide additional detail as to the specific indication of the breakthrough device, the fact that a device intended for use in the field of radiology received a breakthrough device designation is meaningful because FDA's breakthrough device designation program traditionally has not been utilized heavily in the field of radiology. As it can be seen in the graph from FDA, only about 3.1% of the medical devices that received FDA's breakthrough device designation (26 of 839 designations, as of June 30, 2023) were devices in the field of radiology.
There may be several reasons for this apparent underutilization of this pathway, although none offers clear explanation. In fact, according to our analysis, the vast majority (specifically, more than 75% - 392 out of 521 devices) of Artificial Intelligence/Machine Learning-enabled medical devices that FDA cleared, approved, or granted de novo classification for (data current as of October 5, 2022 – the last date of FDA's update) were in the field of radiology. Given that these are medical devices that likely leverage cutting-edge technologies, and have the potential to satisfy the statutory conditions for the breakthrough device designation (laid out in 21 U.S.C. §360e–3(b)), it is not clear why so few of the developers of devices in the radiology field sought breakthrough device designations.
In fact, this trend of underutilization appears to have been true even for the particular type of radiology triage software that Annalise.ai appears to have received the breakthrough device designation for. FDA issued the final order to reclassify this device type (21 C.F.R. 892.2080, "Radiological Computer Aided Triage and Notification Software") from Class III to Class II in January 2020. However, it appears that there have not been any developers that successfully sought the breakthrough device designation until now under this category (although of course, we do not know whether any other developers sought the breakthrough designation in this category).
We view Annalise.ai's achievement as a positive development given that this could serve as a reminder to the industry that breakthrough device designation may be a viable option for their devices. Our hope is that more of the innovative solutions that are being developed in the field of radiology can take advantage of the benefits provided under the breakthrough device designation program.
This article is presented for informational purposes only and is not intended to constitute legal advice.

