In Canada, coverage for prescription drugs currently exists through an array of public and private drug plans. To ensure accessibility of drugs to all Canadians, the federal government is exploring national pharmacare. As part of this effort and as previously reported in our 2019 Federal Budget Life Sciences Highlights, the Advisory Council on the Implementation of National Pharmacare recommended the development of a national formulary, as well as a national drug agency and investment into drug and information technology systems.
In this context, the Canadian Agency for Drugs and Technologies in Health ("CADTH") was mandated to convene an advisory panel (the "Panel") to propose a framework for the development of a potential pan-Canadian drug list. The Panel has recently published a discussion paper with its proposed framework and is inviting stakeholders to provide their input before February 25th, 2022.
Background
On July 27, 2021, an Advisory Panel was convened by CADTH to provide Health Canada with a recommended framework for developing a potential pan-Canadian prescription drug list, or formulary.1
The goal of such a formulary would be to include a broad range of safe, effective, evidence-based drugs and related products that meet the needs of Canada's diverse population.
The Panel's mandate is to:
- recommend principles and a framework for developing a pan-Canadian prescription drug list;
- recommend an initial list of commonly prescribed drugs and a transparent way to add to that list;
- consult with key stakeholders and health system partners - including federal, provincial, and territorial governments; patients, clinicians, industry, and others.2
The Advisory Panel's recommendations are non-binding and are intended to add to the national conversation about ensuring Canadians have access to prescription drugs. The Panel's final report will be submitted to Health Canada and will be shared with provincial and territorial governments.
Overview of the Advisory Panel's Proposed Framework
In early January 2022, the Panel published a discussion paper entitled "Building Toward a Potential Pan-Canadian Formulary", in which its current proposed framework is described. Throughout the paper, stakeholders are asked specific questions aimed at perfecting the draft framework.
The Panel's framework is subdivided in three sections: 1. Formulating the principles for a potential pan-Canadian formulary, 2. Developing a staged approach to creating a potential pan-Canadian formulary, and 3. Exploring opportunities to leverage and enhance existing processes.
Each of these sections is briefly summarized below.
i) Formulating the principles for a potential pan-Canadian formulary
The Panel first recommends six guiding principles around which the potential pan-Canadian formulary should be oriented. These principles are aimed at ensuring that the formulary allows for universal access for all people in Canada across geographic and cultural contexts. The principles are the following:
- Universal and integrated
- Equitable
- Effective and high quality
- Sustainable
- Efficient and timely
- Inclusive, transparent and fair process
The Panel invites stakeholders to comment on the proposed principles and provide suggested changes, if any.
ii) Developing a staged approach to creating a potential formulary
To create the proposed list of commonly prescribed drugs and related products, the Panel suggests a 3-stage approach, which can be summarized as follows:
- Stage 1: Select a small sample list of products as a proof of concept for the process. The sample list should take in consideration therapeutic areas involving drugs with the highest utilization, which diseases are the most significant and growing in prevalence, and which conditions account for high numbers of clinician visits and hospitalizations in Canada. Ensure that the guiding principles are followed while creating the proposed list.
- Stage 2: Review and revise the proposed list as appropriate, then apply the proposed criteria to other therapeutic areas in a subsequent future step to scale the process and expand the proposed list.
- Stage 3: Recommend criteria and processes for adding new drugs and related products once all therapeutic areas have been considered. Also suggest strategies to list over time and to explore how this process could be integrated within the current system.
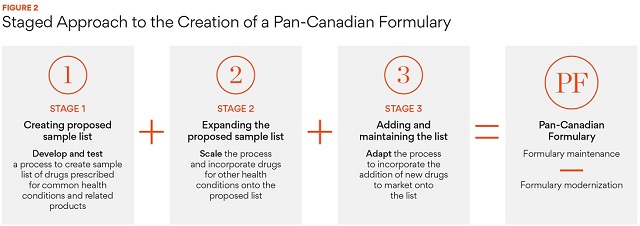
Discussion Paper [PDF], p. 15.
The Panel invites stakeholders to comment on the proposed 3-stage approach.
iii) Exploring opportunities to leverage and enhance existing processes
Finally, the Panel considers that it would be important for the pan-Canadian formulary to work with existing structures and systems. Leveraging existing systems would reduce duplication of processes and provide opportunities to enhance existing processes. For example, CADTH's existing expert review committees could support the evaluation of a new drug or device for a formulary.
Consultation Currently Open
On behalf of the Panel, CADTH invites stakeholders to provide input on all steps of the proposed framework. Stakeholder comments will be used to inform the Panel's final report. The consultation period will close by end of business day on February 25, 2022. The Fasken Life Sciences team has extensive experience in this area and is available to consult with stakeholders in the industry interested in contributing to the discussion.
Footnotes
1 See Advisory Panel to Develop a Framework for a Pan-Canadian Prescription Drug List.
2 See The pan-Canadian Advisory Panel on a Framework for a Prescription Drug List.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


