At the beginning of January 2018, a patent linkage system was promulgated and introduced into the Pharmaceutical Affairs Act. After almost 19 months, Taiwan's Government announced on August 6, 2019 that the patent linkage system shall be implemented from August 20, 2019.
Patent Listing
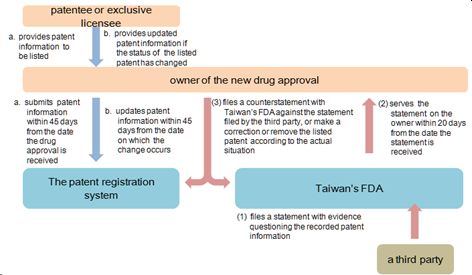
According to the amended Pharmaceutical Affairs Act and related bylaws, to prevent Taiwan's FDA from granting regulatory authorization to a generic medicine, owner of a new drug approval (rather than the patentee) has the right to file an application for recording information relating to a patent to be listed in a patent linkage system run by Taiwan's FDA.
The application must be filed within 45 days from the date the certificate of a new drug approval is received. However, if the certificate of a new drug approval is issued before the patent linkage registration system takes effect, the new drug approval holder is allowed to list its patent(s) by November 20, 2019 (i.e. within three months from the date the amended Pharmaceutical Affairs Act takes effect.)
Only invention patents for compounds, compositions or formulations, as well as medical uses are allowed to be listed:
- Compound: the active ingredient(s) of a drug;
- Composition or formulation: the composition or formulation of the active ingredients of a drug; and
- Medical use: the medical use(s) corresponding to all or part of the indication(s) on the certificate of drug approval.
In general, the information regarding a patent to be listed that need be submitted for recordation in the registration system includes: the patent number and expiry date of the patent, as well as the name of the patent owner, exclusive licensee and patent attorney. For a patent relating to medical use, the number(s) of the Claim(s) corresponding to an indication(s) identified on the certificate of the new drug approval must be specified.
Any changes, including an extension of the patent term, claim amendments, revocation of the patent, and ownership or patent attorney change, must be updated within 45 days from the date when such change occurs.
Public Inspection Proceeding
To prevent abuse of the right of the new drug owner, the patent information recorded in the registration system will be open to the public for inspection. If a third party finds out that any of the recorded information is questionable, such as the listed patent is not related to a compound, composition or formulation, or a medical use, or there is not an update of a listed patent, he may file a statement with Taiwan's FDA in writing along with evidence. Taiwan's FDA will serve a copy of the submitted statement with evidence on the owner of the new drug approval within 20 days from the date the statement and evidence is received. The owner of the new drug approval may file a counterstatement in writing, make a correction, or remove the listed patent within 45 days from the date he receives the statement and evidence from Taiwan's FDA.
It is against the Fair Trade Act and is criminally liable for the owner of a new drug approval to file an application for recording incorrect patent information regarding a listed patent by fraud or deception, with an attempt to prevent generic drugs from obtaining drug approval. Therefore, it is worth noting that all patent information to be recorded should be correct and up-to-date.
Generic Drug Company's Declarations
A generic drug company must make one of the following declarations when filing an application for approval of a generic drug equivalent to a new drug:
- The new drug has not been patented;
- the patent for the new drug has already expired;
- drug approval of the equivalent generic drug is not to be issued until the patent for the new drug has expired; and
- the patent for the new drug is not infringed or is invalid.
If there is no existing patent dispute between a generic drug company and a brand drug company (as shown in the yellow area of the figure), a generic drug company will receive drug approval if declaring (i) or (ii); and will receive drug approval upon expiry of a preceding brand name patent if declaring (iii).
Interplay Between the Patentee and the Generic Drug Company
If declaring (iv), the generic drug company must notify the holder of the new drug approval, the patentee and the exclusive licensee of the patent (if any) of its declaration in writing and with grounds and evidence within 20 days from the day the generic drug company receives a notice from Taiwan FDA that the application for its generic drug has completed.
After receipt of the declaration from the generic drug company, the patentee or the exclusive licensee may file a lawsuit within a 45-day time limit and then notify Taiwan FDA that a lawsuit has been filed.
If a lawsuit is not filed within the time limit, Taiwan FDA will issue drug approval of the generic drug. If a lawsuit is filed, Taiwan FDA will stay issuance of drug approval of the generic drug for up to 12 months.
Should the court render a final and non-appealable judgment in favor of the patentee or the exclusive licensee within the stay period (up to 12 months), the generic drug company will receive drug approval only after the patent for the brand name drug expires. Otherwise, the generic drug company will receive drug approval of the generic drug and further obtain a 12-month market exclusivity, assuming it is the earliest of those that have duly completed the application forms for the generic drug equivalent to the brand name drug.
The details of the patent linkage system are shown in the following figure.

The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
