The U.S. Food and Drug Administration (FDA) has published a new draft guidance titled "Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment." Once finalized, this document will supersede FDA's 2014 guidance titled "Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment" and will complement FDA's 2016 guidance "Assessment of Radiofrequency-Induced Heating in the Magnetic Resonance (MR) Environment for Multi-Configuration Passive Medical Devices."
Although some of the general principles of the original 2014 guidance are retained, the new draft has been significantly expanded to:
- Provide more guidance regarding how to apply the standardized test methods in terms of specifying the worst-case device and when testing is not needed.
- Include considerations specific to electrically active devices.
- Include nonimplanted devices that are expected to enter the MR suite.
- Expand the required content of MR Conditional labeling.
Under the proposed draft guidance, FDA retains the classification terminology of ASTM F2052 of MR Safe, MR Unsafe, and MR Conditional. FDA has clarified the parameters of when devices can be defined as MR safe using a rationale, as opposed to testing, as a device that is electrically nonconductive (defined as conductivity less than 1 S/m), nonmetallic, and nonmagnetic. Most plastics, glass, and many ceramic materials are MR Safe.
In determining what testing must be performed to support the use of a device in the MR environment, the guidance addresses four specific hazards, consistent with the hazards addressed in the current guidance, but FDA has now specified acceptance criteria and worst-case scenarios that should be tested. The four potential risks, their applicability, recommended testing methods, and acceptance criteria are summarized in Table 1, below.
Table 1. Hazards in the MR Environment
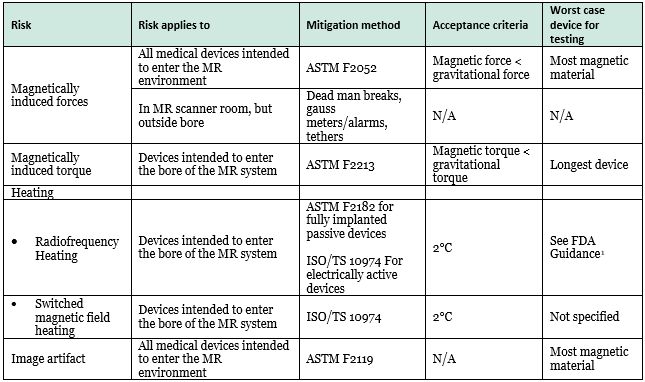
With the expansion of the guidance to include electrically active devices, the guidance identifies several new risks that are specific to these devices that do not apply to passive devices. The new risks include:
- Gradient induced vibration which may lead to device malfunction or tissue damage.
- Unintended stimulation which is caused by the generation of voltage in the electrodes of a fully or partially implanted devices in contact with muscle or nerve tissue.
- Temporary device malfunction during the MR scan or permanent malfunction even after the scan.
- Presence of the active device impact operation of MR system or degrades MR image quality.
Testing to address each of the risks should be performed and FDA refers companies to evaluate these specific risks in accordance with ISO/TS 10974, though, unlike the other tests listed in Table 1 above, it does not define a worst-case or acceptance criteria for these tests noting that in many cases the acceptance criteria will depend on the type of device, where it is located, its intended use, and benefit/risk. Of note, regardless of test outcomes, the draft guidance indicates the electrically active devices should never be labeled as MR Safe due to their electrically conductive components.
As before, FDA expects that results of MR compatibility testing are presented in any FDA marketing application and that pertinent results of the tests be summarized in the device labeling under its own section titled "MRI Safety Information." The draft guidance provides new, recommended language for devices that are MR Unsafe or MR Conditional. The labeling for MR Conditional devices must also either provide the conditions for safe MRI scanning or direct users where this information can be found. The specific safety information depends on if the device is expected to be within the bore of the MR system or not, with FDA proposing inclusion of significantly more detail than the type of information than has previously been required. Additional labeling requirements apply for implanted devices where such devices should be provided with a patient card which identifies the MR safety information.
Importantly, despite the heavy emphasis on risks and testing in the draft guidance, the document proposes to continue allowing companies to proceed to market without evaluating the MR safety of certain devices. Devices with established MR safety, and which are not novel, contain ferromagnetic materials, or are electrically active, may be supported by a rationale regarding the MR safety of the product and accompanied by labeling with a disclosure statement that the device has not been evaluated for safety and compatibility in the MR environment.
In sum, it is clear that FDA has a strong focus on MR safety of medical devices. This is the third version of this guidance to be published in the past 10 years, and FDA has also published related guidance clarifying how to assess radiofrequency heating. This guidance provides more prescriptive information regarding the scope of testing, labeling requirements, and further clarity on when testing may not be necessary and the device can be considered MR Safe. The draft guidance now also extends beyond passive, implanted devices to also address nonimplanted devices in the MR suite and electrically active devices. The guidance will require substantial testing for any electrically active device that may enter the MR suite, either due to its intended use or because it is attached to the patient. Aside for reference to ISO/TS 10974, little guidance such as determination of worst-case or acceptance criteria in regards to how this standard should be applied to active devices is provided as specific risks will be based on the specific use conditions and situations.
FDA is accepting comments to this draft until 1 October 2019 to docket number: FDA-2019-D2837
Footnote
1 "Assessment of Radiofrequency Induced Heating in the Magnetic Resonance (MR) Environment for MultiConfiguration Passive Medical Devices.".
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



