Article 118a of Directive 2001/83/EC requires Member States to lay down "effective, proportionate and dissuasive" penalties for those who are involved in the manufacturing, distribution, brokering, import and export of falsified medicinal products. On 26 January 2018, the European Commission published a report on how this requirement has been met by individual Member States, based on a detailed study performed by external contractors, empirica and ZEIS, on behalf of the DG for Health and Food Safety.
The report indicated that 26 Member States had amended their legislation to accommodate new penalties for the falsification of medicinal products. The exceptions were Hungary, which changed its Criminal Code as a result of the Council of Europe Medicrime Convention, and Finland, which already had penalties in place before Article 118a took effect.
In 21 Member States, the manufacturing, distribution, brokering, import and export of falsified medicinal products attracts criminal penalties. In the remaining Member States (Bulgaria, Finland, Latvia, Romania, Poland, Sweden and Lithuania) only certain infringements are considered criminal.
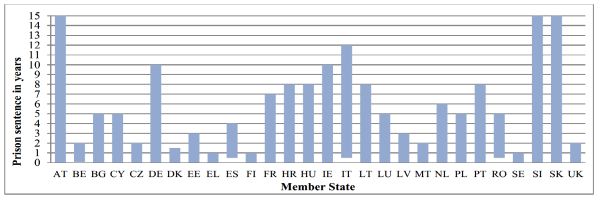
As shown below, maximum prison sentences for those found guilty of falsifying medicinal products range from 1 year in Sweden, Finland and Greece, to 15 years in Austria, Slovenia and Slovakia. In 20 Member States the maximum prison sentence is at least 3 years, which means that such crimes fall under the European Investigation Order, under which Member States must cooperate with and assist each other's competent authorities as they would their own.
The report highlighted significant variance in the maximum fines for those found guilty of falsifying medicinal products, which range from €4,300 in Lithuania, to €1,000,000 in Spain and 'unlimited' in the UK.
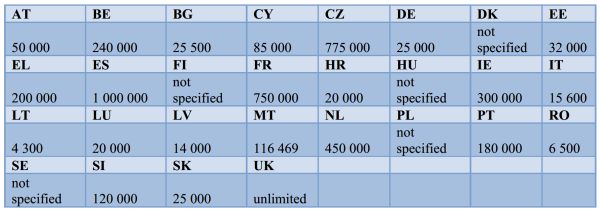
Figure 2 Maximum fines for the falsification of medicinal products (Euros)
All Member States except Finland, Luxembourg and Malta have introduced additional administrative sanctions for the falsification of medicinal products, such as the revocation of licences and restrictions on distribution.
The Commission's report also considered the penalties in respect of breaches of the rules relating to active substances and excipients. In 23 Member States misconduct concerning active substances can attract criminal penalties, but misconduct concerning excipients was only considered a criminal offence in 14 Member States.
Despite the difficulty on obtaining accurate estimates of the extent of falsification in the EU market, experts in 10 Member States commented on the effectiveness of the national measures. There was a general consensus that the penalties in place had "at least some effect" in reducing the presence of falsified medicinal products, with administrative sanctions being rated as effective most often. Experts in 8 Member States provided estimates on the extent to which the penetration of the legal supply chain by falsified medicinal products had been reduced; 6 experts estimated a reduction of more than 25%, whereas 2 experts considered a reduction of less than 5%.
Overall the report concluded that Member States' transposition of Article 118a is "satisfactory". The report urged Member States to ensure that the penalties in place were properly enforced, and that adequate resources and personnel were allocated to such activities. In the meantime the Commission will continue to support the other aspects of the protective measures introduced by the Falsified Medicines Directive, namely the use of the EU logo for online pharmacies and the medicines verification system which is due to come into force in February 2019.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

