On 26 June, the Minister for Health, Simon Harris, signed the Misuse of Drugs (Prescription and Control of Supply of Cannabis for Medical Use) Regulations 2019 (the "2019 Regulations").
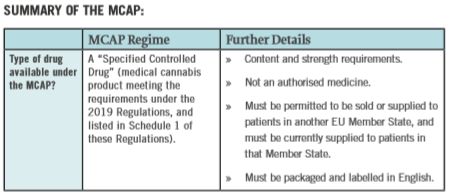
The 2019 Regulations establish the Medical Cannabis Access Programme ("MCAP"), allowing certain patients to be treated with cannabis products (as distinct from authorised medicinal products containing cannabis such as Sativex) and commercial operators to supply these products to the Irish market. The Misuse of Drugs (Designation) (Amendment) Order 2019 and the Misuse of Drugs (Amendment) Regulations 2019, both of which amend existing secondary legislation to facilitate the use of certain cannabis product for medical purposes, were signed into law on the same date.
The MCAP mirrors Denmark's Medicinal Cannabis Pilot Programme, but stops short of permitting the cultivation of cannabis products in Ireland. Although the MCAP is now operational, there are currently no medical cannabis products authorised for supply in Ireland.
Medical Cannabis product manufacturers and suppliers must now apply to the Health Products Regulatory Authority ("HPRA") for the necessary authorisation to make their products available in Ireland. Pending the availability of these products, it is envisaged that health care professionals will continue to use the Ministerial licensing route to access medical cannabis treatments for their patients.


- The application forms can be found on the 'Publications and Forms' section of www.hpra.ie or by contacting controlleddrugs@hpra.ie.
- Once a product is listed by the HPRA in Schedule 1 of the 2019 Regulations, it will automatically be included under Schedule 2 of the Misuse of Drugs Regulations 2017. The 2017 Regulations govern the type of licence required for the import or export of various controlled drugs (now including medical cannabis products).
- https://www.hpra.ie/docs/default-source/publications-forms/guidance-documents/aut-g0127guide-to-import-and-export-licences-andletters-of-no-objection-for-controlled-drugs-v7. pdf?sfvrsn=50 (page 13)
This article contains a general summary of developments and is not a complete or definitive statement of the law. Specific legal advice should be obtained where appropriate.


