The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended five medicines for approval, including two orphan medicines, at its February 2018 meeting23.
A. THE FIVE DRUGS RECOMMENDED FOR APPROVAL ARE:
1. Alpivab (peramivir) - Treatment of uncomplicated influenza
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion and has recommended the granting of a marketing authorization for Alpivab (peramivir)24. Alpivab is indicated for the treatment of uncomplicated influenza in adults and children 2 years and older.
Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms (e.g., fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis). Among children, otitis media, nausea, and vomiting also are commonly reported with influenza illness. However, influenza virus infections can cause primary influenza viral pneumonia; exacerbate underlying medical conditions (e.g., pulmonary or cardiac disease); lead to secondary bacterial pneumonia, sinusitis, or otitis media; or contribute to coinfections with other viral or bacterial pathogens25.
Alpivab is an inhibitor of influenza virus neuraminidase, an enzyme important for viral entry into uninfected cells and release and spread of new virus once cells have been infected. The benefits with Alpivab are its ability to speed alleviation of symptoms and recovery of normal temperature in patients with uncomplicated influenza. The most common side effects are gastro-intestinal disorders, such as diarrhea and vomiting.
The applicant for Alpivab is Biocryst UK Limited.
2. Mylotarg (gemtuzumab ozogamicin) - Treatment of acute myeloid leukaemia
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended the granting of a marketing authorization for Mylotarg26, indicated for combination therapy with daunorubicin (DNR) and cytarabine (AraC) for the treatment of patients age 15 years and above with previously untreated, de novo CD33-positive acute myeloid leukaemia (AML), except acute promyelocytic leukaemia (APL)
Mylotarg is a humanized immunoglobulin G subtype 4 (IgG4) antibody directed at CD33 which is conjugated to calicheamicin, a toxin which induces breaks in double-stranded DNA, subsequently inducing cell cycle arrest and apoptotic cell death. The benefit with Mylotarg is improvement in event-free survival.
The applicant for Mylotarg is Pfizer Limited.
3. Amglidia (glibenclamide) – First medicine to treat neonatal Diabetes
The CHMP has recommended granting a marketing authorization in the European Union (EU) for Amglidia (glibenclamide)27, a medicine indicated for the treatment of neonatal diabetes mellitus (NDM), for use in newborns, infants and children.
Neonatal diabetes is an extremely rare form of diabetes that is diagnosed in the first six months of life. It is life- threatening and debilitating because of the symptoms caused by high blood sugar levels and the risk of ketoacidosis, a serious problem that can occur in people with diabetes if their body starts to run out of insulin and ketones build up in the body. Different gene mutations have been identified which cause this type of diabetes.
Amglidia is a new oral formulation of glibenclamide, a medicine which is already authorized for treating type 2 diabetes, specifically developed for use in newborns, toddlers and children with neonatal diabetes. It works on insulin-producing cells in the pancreas by attaching to an ATP-sensitive potassium (KATP) channel, which controls the release of insulin. In many newborn babies with neonatal diabetes, the cells in the pancreas produce insulin but they are not able to release it into the blood because their gene mutations lead to dysfunctional KATP channels.
The applicant for Amglidia is AMMTeK.
4. Riarify - Maintenance treatment of adult patients with moderate to severe chronic obstructive pulmonary disease (COPD)
The EMA's CHMP has recommended the granting of a marketing authorization for Riarify28, intended for the maintenance treatment of adult patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Riarify is a triple combination of an inhaled glucocorticoid (beclometasone dipropionate), a long-acting beta-2 receptor agonist (formoterol fumarate dihydrate) and a long-acting muscarinic antagonist (glycopyrronium bromide). It will be available as a pressurized metered dose inhaler delivering a solution with a nominal dose per actuation of 87 micrograms / 5 micrograms / 9 micrograms of the active substances respectively. Beclometasone reduces inflammation in the lungs, whereas formoterol and glycopyrronium relax bronchial smooth muscle helping to dilate the airways and make breathing easier. The benefits with Riarify are its ability to relieve and prevent symptoms such as shortness of breath, wheezing and cough and to reduce exacerbations of COPD symptoms.
The applicant for Riarify is Chiesi Farmaceutici S.p.A.
5. Trydonis - Maintenance treatment of adult patients with moderate to severe chronic obstructive pulmonary disease (COPD)
The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended the granting of a marketing authorization for the medicinal product Trydonis29, intended for the maintenance treatment of adult patients with moderate to severe chronic obstructive pulmonary disease (COPD).
Trydonis is a triple combination of an inhaled glucocorticoid (beclometasone dipropionate), a long-acting beta-2 receptor agonist (formoterol fumarate dihydrate) and a long-acting muscarinic antagonist (glycopyrronium bromide). It will be available as a pressurized metered dose inhaler delivering a solution with a nominal dose per actuation of 87 micrograms / 5 micrograms / 9 micrograms of the active substances respectively. Beclometasone reduces inflammation in the lungs, whereas formoterol and glycopyrronium relax bronchial smooth muscle helping to dilate the airways and make breathing easier. The benefits with Trydonis are its ability to relieve and prevent symptoms such as shortness of breath, wheezing and cough and to reduce exacerbations of COPD symptoms.
The applicant for this medicinal product is Chiesi Farmaceutici S.p.A.
B. NEGATIVE OPINION ON TWO MEDICINES:
1. Nerlynx (neratinib) – Intended to treat breast cancer
The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended the refusal of marketing authorization for Nerlynx30, intended for the treatment of breast cancer.
Nerlynx is a tyrosine kinase inhibitor, a cancer medicine. It attaches to the HER2 protein on the cancer cells, and so blocks its action. Because HER2 helps cancer cells to grow and divide, blocking it helps to stop them growing and prevent the cancer from coming back.
The CHMP considered that though a greater proportion of women given Nerlynx in the study lived for 2 years without their disease coming back than women given placebo (around 94% versus 92% respectively); it is uncertain that this difference in benefit would be seen in clinical practice. Furthermore, Nerlynx causes side effects in the digestive system, particularly diarrhoea, which affected most patients and might be difficult to manage. The Committee therefore, concluded that the benefits were not enough to outweigh the risk of side effects and recommended that Nerlynx be refused marketing authorization.
Application for authorization for Nerlynx was made by Puma Biotechnology Ltd.
2. Sutent (sunitinib) - Expected to be used to delay or prevent the return of kidney cancer
The EMA's CHMP has recommended the refusal of a change to the marketing authorization for Sutent31. The CHMP considered that the evidence that Sutent delays the return of cancer was not convincing. Given the known side effects of the medicine, the Committee concluded that the benefits did not outweigh the risks and recommended that the change to the marketing authorization of Sutent be refused.
Sutent is a protein kinase inhibitor. This means that it blocks some specific enzymes known as protein kinases involved in the growth and spread of cancer cells and the development of new blood vessels supplying them. By blocking these enzymes, Sutent can reduce the growth and spread of cancer and cut off the blood supply that keeps cancer cells growing.
The company that applied for the change to the authorization is Pfizer Limited.
C. SIX RECOMMENDATIONS ON EXTENSIONS OF THERAPEUTIC INDICATION:
The CHMP has recommended a change to the terms of the marketing authorization for six drugs on extensions of therapeutic indication as described in table-
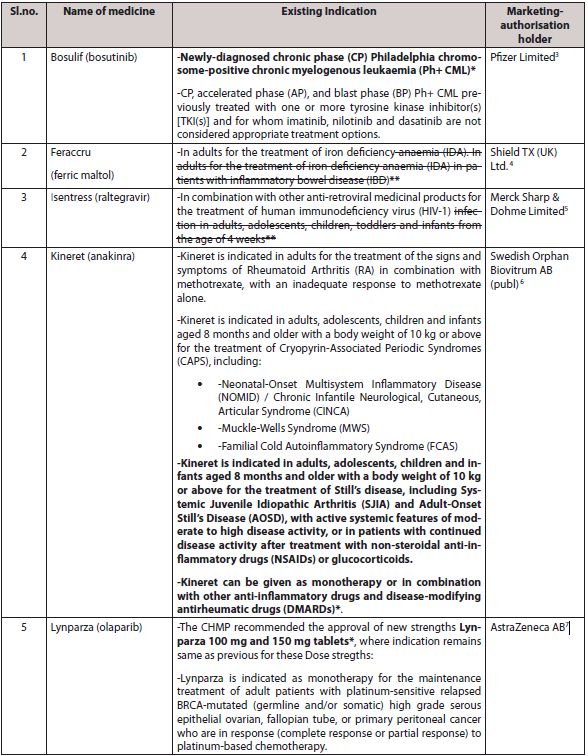

D. WITHDRAWAL OF APPLICATION FOR EXTENSION OF INDICATION
1. Zydelig (idelalisib) – Treatment of chronic lymphocytic leukaemia (CLL) and follicular lymphoma
Gilead Sciences International Ltd. officially notified the Committee for Medicinal Products for Human Use (CHMP) that it wishes to withdraw its application to use the cancer medicine Zydelig in combination with the cancer medicines rituximab and bendamustine for the treatment of chronic lymphocytic leukaemia (CLL).
Zydelig blocks the effects of an enzyme called PI3K-delta. This enzyme plays a role in the growth, migration and survival of white blood cells but is overactive in blood cancers, where it enables the survival of the cancer cells. By targeting this enzyme and blocking its effects, idelalisib causes death of the cancer cells, thereby delaying or stopping the progression of the cancer.
The CHMP noted that patients treated with Zydelig in addition to rituximab and bendamustine lived longer without their disease getting worse than those receiving placebo in addition to rituximab and bendamustine. However, because of the design of the study and the side effect profile of Zydelig, the CHMP considered that longer term data was needed to show that the benefits of Zydelig outweighed its risks in this combination32.
Footnotes
25. https://www.cdc.gov/flu/professionals/acip/clinical.htm
31. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000687/WC500244293.pdf
32. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2018/02/WC500244301.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

