In May, the Trump Administration announced its Blueprint to Lower Drug Prices and HHS Secretary Azar issued a Request for Information seeking comments from interested parties "to help shape future policy development and agency action" related to drug pricing issues. Since that time, many industry commentators questioned whether any of the vague goals in the Blueprint would or could be turned into concrete action.

This month, the Trump Administration has attempted to answer that question through a variety of HHS and FDA initiatives. Here's what you need to know about drug pricing developments so far in July.
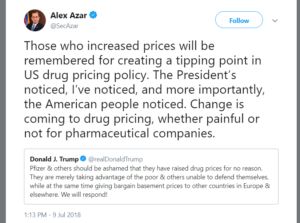
- July 9, 2018 – President Trump and Secretary Azar criticize manufacturer drug price increases and foreshadow July drug pricing developments.
- July 10, 2018 – Pfizer agrees to postpone drug price increases.


- July 12, 2018 – CMS releases a proposed rule to reduce payments for new drugs under the Medicare Part B program. Click here to read our blog post with details on and analysis of this proposed rule.
- July 12, 2018 – FDA commissioner Scott Gottlieb announces new efforts to advance medical product communications to support drug competition and value-based health care. The Trump Administration Blueprint identifies as priorities (i) the of linking payments for drugs to their value and (ii) the removal of regulatory obstacles to value-based purchasing by payors. Commissioner Gottlied states "To advance these goals, the FDA is issuing updated, final guidance documents that provide greater clarity about our thinking and recommendations for certain medical product communications. In particular, this guidance will inform market participants developing contracts that include value-based arrangements how to communicate information about how a drug might impact outcomes that are important to purchasers like a health plan or hospital, but is not an endpoint that is expressly described in the drug's approved labeling."
- July 16, 2018 – Comments are due on the drug pricing RFI and HHS announces that it received over 3,000 comments. Click here to read comments from PhRMA and here for comments from PCMA.
- July 17, 2018 – FDA Commissioner Gottlieb announces new efforts to increase access to non-prescription drug products.
- July 18, 2018 – FDA issues a Biosimilars Action Plan intended to promote competition and affordability for biologics and biosimilar products. Read our blog post here for more details on the Biosimilars Action Plan.

- July 18, 2018 – Novartis announces on an investor call it will not raise prices on its products in the United States for the rest of 2018.
- July 18, 2018 – Office of Information and Regulatory Affairs (OIRA) receives for review from HHS-OIG a proposed rule entitled "Removal Of Safe Harbor Protection for Rebates to Plans or PBMs Involving Prescription Pharmaceuticals and Creation of New Safe Harbor Protection." The Administration has identified removal of anti-kickback statute safe harbor protection for drug rebates paid to insurers and PBMs as a potential strategy to address high drug prices and HHS's Blueprint specifically requests industry comments on the impact such a change could have on the high list prices of drugs. It is worth noting that the title of the regulation indicates that OIG will be proposing new safe harbor protection for formulary rebates. However, as our ML Strategies colleagues note, the details of how HHS-OIG would effectuate such a change remain to be seen and the comments to HHS's Blueprint RFI suggest that such a change will not be without challenge. You can read more about the 14 pending regulations under review by OIRA in our weekly ML Strategies Health Care Preview.
![]()
- July 19, 2018 – Secretary Azar directs FDA to establish a working group to examine how to safely import prescription drugs from other countries in the event of a significant drug price increase for a drug produced by one manufacturer and not protected by patents or exclusivities.
- July 19, 2018 – Merck announces it will cut U.S. list prices for several of its drugs including the hepatitis C treatment Zepatier, and pledges to limit future net price increases. Industry analysts are skeptical about the the scope of these manufacturer pricing actions.
- July 20, 2018 – Azar takes to Twitter to tout the Administration's recent actions. Click here to view Secretary Azar's full tweet outlining recent agency actions and developments.

- July 23, 2018 – Secretary Azar directs CMS Administrator Seema Verma to examine how Medicare Part D plans can further encourage the use of generics instead of branded products, noting that a recent HHS report found that Medicare paid $9 billion on brand drugs where a therapeutically equivalent generic drug was available.
- July 24, 2018 – CMS issues a memo to Medicare Part D plans explaining CMS's expectations regarding formulary exception requests and the tools available to plans to ensure that beneficiaries utilize low-cost generics when available on formulary.
There are only 7 days left in July, but we will be keeping an eye out for new developments and, in particular, for any indication of movement on the proposed rule relating to discount safe harbor protection for formulary rebates. Stay tuned!
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

